
What would be the product formed when 1−bromo−3−chlorocyclobutane reacts with two equivalents of metallic sodium in the ether?
A . 1-Bromocyclobutane
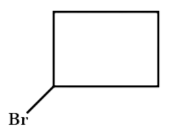
B . 1 - Chlorocyclobutane
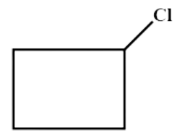
C . Cyclobutane

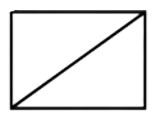
D . Bicyclo ( 1 , 1 , 0 ) ) butane
Answer
231.9k+ views
Hint: In this question, intramolecular Wurtz reaction is taking place. With the help of radical species R, the Wurtz reaction entails the transfer of halogen and metal in order to create a carbon-carbon bond that results from a nucleophilic substitution process. The process involves reacting alkyl halides with metallic sodium in the presence of dry ether to produce higher alkanes.
Wurtz reaction equation:
R-X + 2Na + X-R→ R–R + 2NaX, where X = halogen (Cl, Br, I)
Complete step by step solution:
Given that 1−bromo−3−chlorocyclobutane reacts with two equivalents of metallic sodium in the ether.
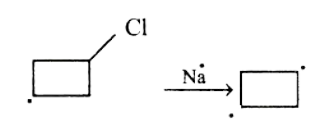
In the first step, Na will attack the Bromine-Carbon bond and a carbanion will be formed on the chlorocyclobutane.
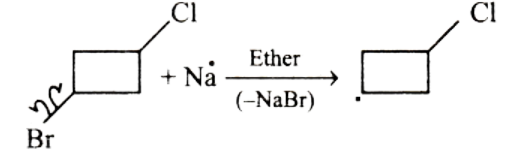
After the formation of carbanion, Na will attack the Chlorine-Carbon bond and another carbanion will be formed on the chlorocyclobutane.
The formation of two carbanions on a 4-membered ring is very unstable and hence the two carbanions join to form a bond, and thus form Bicyclo ( 1 , 1 , 0 ) butane

Hence, the overall chemical reaction when 1−bromo−3−chlorocyclobutane reacts with two equivalents of metallic sodium in the ether is:
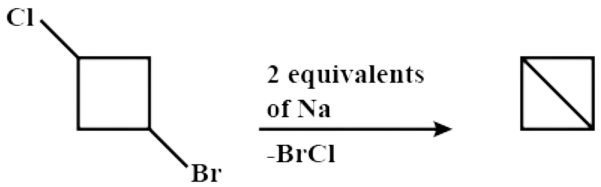
Thus, Option (D) is correct.
Note: A BrCl molecule is removed in this intramolecular Wurtz reaction, and a Carbon-Carbon bond is created, producing a bicyclic compound. The order of reactivity of alkyl halides in Wurtz reaction is R-I(alkyl iodide) >R-Br(alkyl bromide) >R-Cl(alkyl chloride), because Carbon-Bromine bond is weaker than Carbon-Chlorine bond. Hence. Wurtz reaction will take place with C-Br bond in the first step.
Wurtz reaction equation:
R-X + 2Na + X-R→ R–R + 2NaX, where X = halogen (Cl, Br, I)
Complete step by step solution:
Given that 1−bromo−3−chlorocyclobutane reacts with two equivalents of metallic sodium in the ether.
In the first step, Na will attack the Bromine-Carbon bond and a carbanion will be formed on the chlorocyclobutane.
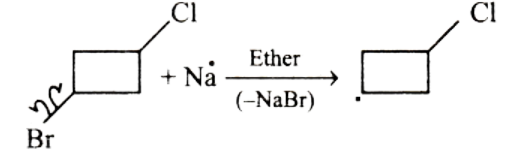
After the formation of carbanion, Na will attack the Chlorine-Carbon bond and another carbanion will be formed on the chlorocyclobutane.

The formation of two carbanions on a 4-membered ring is very unstable and hence the two carbanions join to form a bond, and thus form Bicyclo ( 1 , 1 , 0 ) butane

Hence, the overall chemical reaction when 1−bromo−3−chlorocyclobutane reacts with two equivalents of metallic sodium in the ether is:

Thus, Option (D) is correct.
Note: A BrCl molecule is removed in this intramolecular Wurtz reaction, and a Carbon-Carbon bond is created, producing a bicyclic compound. The order of reactivity of alkyl halides in Wurtz reaction is R-I(alkyl iodide) >R-Br(alkyl bromide) >R-Cl(alkyl chloride), because Carbon-Bromine bond is weaker than Carbon-Chlorine bond. Hence. Wurtz reaction will take place with C-Br bond in the first step.
Recently Updated Pages
Chlorobenzene is extremely less reactive towards a class 12 chemistry JEE_Main

How many dichlorocyclohexane would be obtained on chlorination class 12 chemistry JEE_Main

The vapour pressure of pure A is 10 torr and at the class 12 chemistry JEE_Main

An alcohol A gives Lucas test within 5 minutes 74 g class 12 chemistry JEE_Main

Which one of the following statements is not true 1 class 12 chemistry JEE_Main

Ethene when treated with Br2 in the presence of CCl4 class 12 chemistry JEE_Main

Trending doubts
JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding Electromagnetic Waves and Their Importance

Inductive Effect and Its Role in Acidic Strength

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding Average and RMS Value in Electrical Circuits

Other Pages
JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Understanding Collisions: Types and Examples for Students

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

JEE Advanced 2026 Revision Notes for Practical Organic Chemistry

NCERT Solutions For Class 12 Chemistry Chapter 2 Solutions Hindi Medium (2025-26)




