
Give the IUPAC name of $C{H_3}C{H_2}C{H_2}CHO$.
Answer
523.5k+ views
Hint: The given compound $C{H_3}C{H_2}C{H_2}CHO$ is an aldehyde as –CHO group is attached to the carbon which represents the functional group aldehyde. The carbon is a tetravalent compound so its valency is fulfilled as it is bonded with four other groups.
Complete step by step answer:
There are some guidelines set by the International Union of Pure and Applied Chemistry for determining the name for any chemical compound which is known as IUPAC name. By following those rules, the IUPAC name of $C{H_3}C{H_2}C{H_2}CHO$ is determined as follows.
The structure of the given compound is shown below.
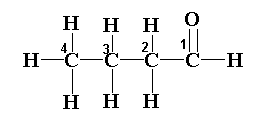
STEP 1: Identify the group
In the given hydrocarbon, an aldehyde group is present at the first position. Therefore the suffix will be given as –al.
STEP 2: Find the longest carbon chain
The hydrocarbon consists of four carbon atoms in the longest chain or parent chain. Therefore, the hydrocarbon is named as butane.
STEP 3: Number the carbon atoms present in the longest chain
The numbering of the carbon atom is shown in the diagram. The numbering will start from the highest priority group that is the functional group in this compound.
STEP 4: Combine the entire element into a single IUPAC name
The IUPAC name of the compound $C{H_3}C{H_2}C{H_2}CHO$ is butanal.
Note:
In this compound, the numbering starts from the carbon of the aldehyde group as it is included in the hydrocarbon chain. If any other group or functional group is present where carbon is not one of the atoms, then numbering starts from the first carbon present adjacent to the functional group.
Complete step by step answer:
There are some guidelines set by the International Union of Pure and Applied Chemistry for determining the name for any chemical compound which is known as IUPAC name. By following those rules, the IUPAC name of $C{H_3}C{H_2}C{H_2}CHO$ is determined as follows.
The structure of the given compound is shown below.

STEP 1: Identify the group
In the given hydrocarbon, an aldehyde group is present at the first position. Therefore the suffix will be given as –al.
STEP 2: Find the longest carbon chain
The hydrocarbon consists of four carbon atoms in the longest chain or parent chain. Therefore, the hydrocarbon is named as butane.
STEP 3: Number the carbon atoms present in the longest chain
The numbering of the carbon atom is shown in the diagram. The numbering will start from the highest priority group that is the functional group in this compound.
STEP 4: Combine the entire element into a single IUPAC name
The IUPAC name of the compound $C{H_3}C{H_2}C{H_2}CHO$ is butanal.
Note:
In this compound, the numbering starts from the carbon of the aldehyde group as it is included in the hydrocarbon chain. If any other group or functional group is present where carbon is not one of the atoms, then numbering starts from the first carbon present adjacent to the functional group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




