
What is 1s 2s 2p 3s 3p ?
Answer
507.3k+ views
Hint: The electrons revolve around the nucleus in fixed energy shells or orbits. This concept was first proposed by Niels Bohr in 1913.These electrons can be moved to either high or low energy levels simply by absorbing or releasing fixed amounts of energy. Thus, we can say that this entire system is quantized (having a fixed amount of energy). An orbital represents a 3-dimensional space around the atomic nucleus where probability of finding an electron is maximum (approximately 95%).
Complete answer:
The electrons in the shell closest to the nucleus will have lowest energy while at higher levels it will have higher energy. Each shell can accommodate $2{n^2}$ electrons. So, the first level can have 2 electrons and corresponds to s orbital, the second level can have 8 electrons with s and p orbital both and so on. Within each orbit or shell, we have different combinations of orbitals. There are 4 types of orbitals-s(sharp), p(principle), d(diffuse) and f(fundamental).
To completely describe an electron in an atom we have 4 quantum numbers-principal $(n)$, Azimuthal $(l)$, magnetic $({m_l})$ and spin $({m_s})$ quantum numbers.
In the question 1s 2s 2p 3s 3p represents electron orbital energy levels. These orbital energy levels depend on 2 quantum numbers-Principal quantum number $(n)$ and Azimuthal quantum number$(l)$ .Principal quantum number describe the electronic shell of an atom. It describes the distance between the nucleus and the electron. Here n can have values like $n = 1,2,3,4....$etc. $n = 1$ means the shell or orbit closest to the nucleus. Azimuthal or orbital angular momentum quantum number describes the shape of orbitals of that atom. Its value is $0$ to $(n - 1)$.
The electron orbital energy level is arranged as follows-
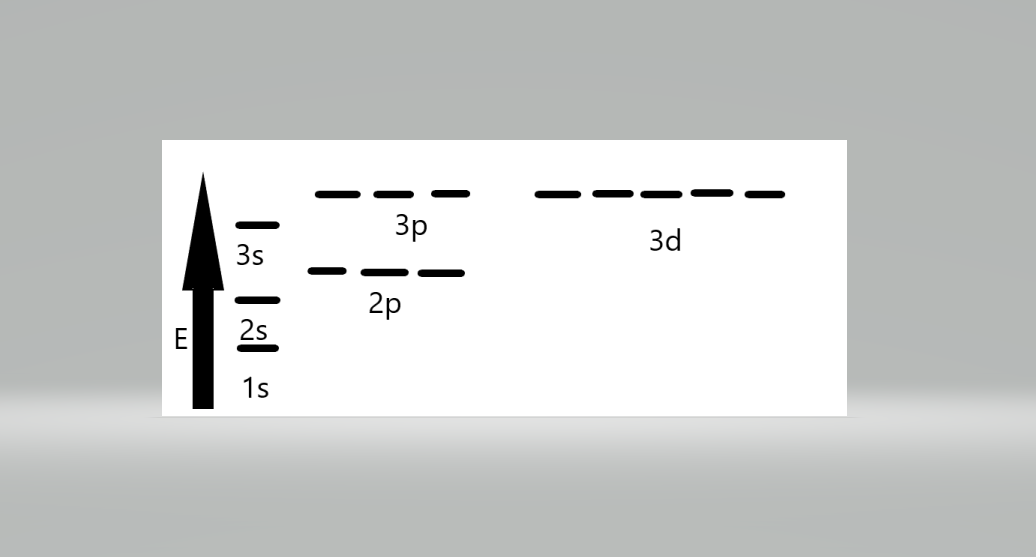
The sequence of orbital energy levels is as always-1s < 2s = 2p < 3s = 3p = 3d <4s = 4p = 4d= 4f. The orbital having the same energy is called a degenerate orbital.
Note:
The difference in the energy of electrons is mainly due to the repulsive forces acting between them. So apart from the attraction type of force between nucleus and electron we have a repulsive force between electrons and to maintain the stability of an atom attractive force must be greater than the repulsive force. The attraction being highest for the nearest shell electron and least for the outermost shell electron.
Complete answer:
The electrons in the shell closest to the nucleus will have lowest energy while at higher levels it will have higher energy. Each shell can accommodate $2{n^2}$ electrons. So, the first level can have 2 electrons and corresponds to s orbital, the second level can have 8 electrons with s and p orbital both and so on. Within each orbit or shell, we have different combinations of orbitals. There are 4 types of orbitals-s(sharp), p(principle), d(diffuse) and f(fundamental).
To completely describe an electron in an atom we have 4 quantum numbers-principal $(n)$, Azimuthal $(l)$, magnetic $({m_l})$ and spin $({m_s})$ quantum numbers.
In the question 1s 2s 2p 3s 3p represents electron orbital energy levels. These orbital energy levels depend on 2 quantum numbers-Principal quantum number $(n)$ and Azimuthal quantum number$(l)$ .Principal quantum number describe the electronic shell of an atom. It describes the distance between the nucleus and the electron. Here n can have values like $n = 1,2,3,4....$etc. $n = 1$ means the shell or orbit closest to the nucleus. Azimuthal or orbital angular momentum quantum number describes the shape of orbitals of that atom. Its value is $0$ to $(n - 1)$.
| Azimuthal quantum number(l) | Orbital |
| 0 | s |
| 1 | p |
| 2 | d |
| 3 | f |
The electron orbital energy level is arranged as follows-

The sequence of orbital energy levels is as always-1s < 2s = 2p < 3s = 3p = 3d <4s = 4p = 4d= 4f. The orbital having the same energy is called a degenerate orbital.
Note:
The difference in the energy of electrons is mainly due to the repulsive forces acting between them. So apart from the attraction type of force between nucleus and electron we have a repulsive force between electrons and to maintain the stability of an atom attractive force must be greater than the repulsive force. The attraction being highest for the nearest shell electron and least for the outermost shell electron.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




