




Real-Life Examples of Thermal Equilibrium and How It Works
Thermal equilibrium is a core concept in physics, revealing how energy flows and balances within and between systems. Understanding this principle is crucial for topics like thermodynamics, statistical physics, and real-world applications such as climate, engines, and even MRI machines.
Thermal Equilibrium: Simple Definition and Physical Meaning
Thermal equilibrium simple definition: it is the state where two or more systems in thermal contact no longer transfer heat between each other because their temperatures are equal. Physical intuition for this concept comes from daily life—when you drop an ice cube in a drink, energy moves from the warmer liquid to the colder ice, but as both reach the same temperature, energy exchange ceases.
This idea forms the backbone of the thermal equilibrium definition in physics: thermal equilibrium means no temperature gradient exists between objects in contact, so there is no net flow of heat. This uniformity ensures further energetic changes do not spontaneously occur, stabilizing the system's physical properties.
Microscopic Mechanism and Conceptual Analogy
At the microscopic level, thermal equilibrium results from random particle interactions. When two materials connect thermally, particles with higher energies from the warmer object transfer energy to lower-energy particles in the cooler one, rapidly equalizing kinetic energies.
For example, consider two pieces of metal, one hot and one cold. When joined, their atoms vibrate and collide at the interface, passing energy until both objects settle at a common temperature. A misconception is that energy stops moving altogether in equilibrium, but actually, individual molecules always move; it is just that net energy flow is zero. This is much like two equally full water tanks connected by a pipe: water molecules still move, but with no net flow of water.
Thermal Equilibrium Equation: Understanding the Balance
The thermal equilibrium equation captures the balance of energy: the total heat lost by hotter bodies equals the total heat gained by cooler ones. Mathematically, this is expressed as:
Qlost + Qgained = 0, or for two bodies: m1C1(Tf – T1) + m2C2(Tf – T2) = 0.
Here, m is mass, C is specific heat capacity, Tf is final equilibrium temperature, and T1, T2 are initial temperatures. This relation is the foundation for solving calorimetry problems—a favorite for JEE, often requiring careful interpretation or evaluation of unknowns.
Thermal Equilibrium Examples and Conceptual Micro-Examples
One classic example of thermal equilibrium is a spoon placed in a hot cup of tea. Over time, the cooler spoon absorbs heat until both reach the same temperature, and no further heat exchange occurs. JEE often frames such scenarios, probing your understanding of energy flow direction, not just magnitude. Another example is Earth’s surface and atmosphere: for global temperatures to stay steady, energy radiated by the Earth must balance with incoming solar energy—a large-scale thermal equilibrium example.
When two objects—say, a copper block and an aluminum block—are placed together and insulated from the surroundings, their temperatures eventually equalize, reaching thermal equilibrium. In such cases, a thermal equilibrium calculator can help estimate the final temperature using the thermal equilibrium equation.

A common misconception is that all forms of equilibrium are the same, but thermal equilibrium concerns only temperature. Mechanical or chemical equilibrium, involving force or composition, require separate scrutiny—even if a system is in one, it may not be in another.
Equilibrium Types and Thermodynamic Context
Thermal equilibrium is only one aspect of a system’s overall state. In thermodynamics, three principal types are distinguished to understand a system’s stability and predict its response to external disturbances:
- Thermal equilibrium—uniform temperature, no net heat flow
- Mechanical equilibrium—uniform pressure, no net forces
- Chemical equilibrium—no change in chemical composition
In the context of a bottle of water at room temperature, the water is at thermal equilibrium with its environment, mechanical equilibrium with the atmosphere, and chemical equilibrium within itself. All these must hold for the system to be in complete thermodynamic equilibrium. JEE sometimes checks if students confuse these types, though each has a distinct definition and implication.

Some assume temperature variations must be large to break equilibrium, but even tiny differences drive heat flow. True equilibrium exists only when even microscopic gradients vanish.
Zeroth Law of Thermodynamics and Its Role
Thermal equilibrium meaning in physics is formalized by the Zeroth Law of Thermodynamics. This law states: if system A is in thermal equilibrium with system B, and B is in equilibrium with system C, then A is also in equilibrium with C. This property underpins everyday temperature measurement: if your thermometer comes into thermal equilibrium with your body, its reading matches your body's temperature. The law guarantees that temperature is a valid, universal property of matter.
JEE exams may require you to apply the Zeroth Law conceptually or use it to determine direction of energy flow in multi-object systems. A misconception is that thermometers always instantly give correct readings, but actually, they must reach full thermal equilibrium with the measured object.
Thermal Equilibrium in Chemistry and Advanced Physics
In chemistry, the thermal equilibrium definition chemistry is similar: no temperature gradients in a system, so no net energy transfer. This creates ideal conditions for studying reaction rates or balance between phases—solids, liquids, gases—since temperature directly impacts molecular speeds and interactions.
Thermal equilibrium meaning also extends to contexts like MRI scanners. In MRI physics, the equilibrium refers to nuclear spins in tissues redistributing their energy via surroundings, so the system returns to its ground state after being disturbed by radio waves. JEE rarely touches on MRI, but understanding this variant helps appreciate the broad significance of equilibrium in technology and biomedicine.
Table: Comparison of Equilibrium Types
| Equilibrium Type | Key Criterion |
|---|---|
| Thermal Equilibrium | Uniform temperature, no net heat flow |
| Mechanical Equilibrium | Uniform pressure, no net force |
| Chemical Equilibrium | Constant composition, no net reactions |
Role in Real-World and Engineering Applications
Applications of thermal equilibrium span engineering, meteorology, and daily life. For instance, modern engines rely on design principles that manage heat flow efficiently, swiftly bringing components into thermal equilibrium to maximize performance and prevent damage. Similarly, climate models depend on Earth's radiative thermal equilibrium with the solar input for accurate temperature predictions.
In electronics, even heat sinks operate on this principle, drawing thermal energy away from delicate parts until equilibrium is set with the ambient environment. Thermal equilibrium calculators help engineers optimize such systems by balancing material properties and environmental factors. A common misconception is thinking only large or hot systems matter, when in fact, even tiny circuits or molecules depend on these energy principles.
Sample Sentences and Everyday Intuition
Thermal equilibrium in a sentence: “After waiting a few minutes, the thermometer displayed room temperature, showing it had reached thermal equilibrium with the air.” For more intuition, think about how the metal body of a car feels hot in the sun, but cools down to match night air temperature by morning—a clear example of natural thermal equilibrium.
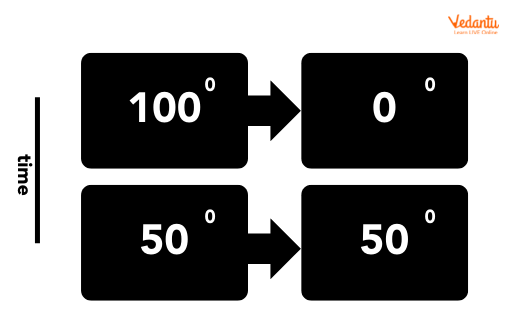
JEE problems can sometimes twist everyday cases, flipping the environment or changing the scale, ensuring you really understand how equilibrium arguments work in diverse circumstances. Using Vedantu's curated question banks sharpens this skill for exams.
Misconceptions and Subtle Points
A recurring misconception is that once systems stop transferring heat, all molecular motion halts. In fact, particles remain in constant thermal motion—just balanced. Also, it's easy to mix up equilibrium types. Remember: thermal equilibrium only means equal temperatures; nothing about force or chemical change is implied.
JEE sometimes probes if students misapply concepts—such as assuming mechanical equilibrium also means thermal equilibrium. Careful reading and tracing which property is under consideration can avoid common mistakes. Dimensional consistency of the thermal equilibrium equation is also a powerful tool for checking work and eliminating unreasonable answers.
Key Takeaways and How to Master Thermal Equilibrium for JEE
The thermal equilibrium meaning is foundational in thermodynamics, connecting temperature, energy transfer, and system stability. Mastering the thermal equilibrium equation, distinguishing equilibrium types, and learning to apply the concept across physics and chemistry are essential for JEE success.
Regularly practicing with thermal equilibrium examples, carefully analyzing micro-examples, and working through Vedantu’s targeted resources will sharpen both conceptual understanding and problem-solving accuracy, which is crucial for tackling the unexpected twists in JEE physics questions.
To deepen your knowledge, you can also explore related concepts such as Thermal Expansion Explained and the Zeroth Law of Thermodynamics for broader insight into equilibrium states.
By internalizing the principles of thermal equilibrium and recognizing its real-world manifestations, you build a strong platform for higher studies and practical problem solving in physics and engineering.
FAQs on What Is Thermal Equilibrium?
1. What is thermal equilibrium?
Thermal equilibrium is a state where two or more objects in contact have no net flow of heat energy between them.
- This means all objects have reached the same temperature.
- There is no further heat transfer between the bodies.
- The concept is essential for understanding thermodynamics and temperature measurement.
2. What are the conditions for thermal equilibrium?
Thermal equilibrium occurs when certain conditions are met:
- All objects in contact have the same temperature.
- There is no net exchange of heat energy between them.
- The system is isolated from external heat sources.
3. Why is thermal equilibrium important in thermodynamics?
In thermodynamics, thermal equilibrium is crucial because it defines a stable state where properties like temperature can be measured accurately.
- It forms the basis for the definition of temperature.
- It is essential for formulating thermodynamic laws like the zeroth law.
4. State the zeroth law of thermodynamics.
The zeroth law of thermodynamics states:
If body A is in thermal equilibrium with body B, and body B is in thermal equilibrium with body C, then body A is also in thermal equilibrium with body C.
- This law allows for the definition of temperature as a property that determines thermal equilibrium.
5. What happens if two bodies are not in thermal equilibrium?
If two bodies are not in thermal equilibrium, heat flows from the object at higher temperature to the one at lower temperature until equilibrium is reached.
- There will be a continuous exchange of thermal energy.
- Their temperatures gradually become equal.
6. How is temperature related to thermal equilibrium?
Temperature is the physical quantity that determines whether two bodies are in thermal equilibrium.
- If their temperatures are equal, no heat flows between them.
- Temperature defines the state of thermal equilibrium between objects.
7. Give an example of thermal equilibrium in daily life.
A common example of thermal equilibrium is when a cup of hot tea is left in a room, it eventually reaches the same temperature as the room air.
- Heat transfers from hot tea to the cooler air until they are at the same temperature.
- No further heat exchange occurs afterwards.
8. What is the difference between thermal equilibrium and thermal non-equilibrium?
Thermal equilibrium means all objects have the same temperature and no heat flows between them, whereas thermal non-equilibrium occurs when objects have different temperatures and heat transfer takes place.
- Thermal equilibrium = No net heat flow.
- Thermal non-equilibrium = Heat flows from higher to lower temperature.
9. What role does thermal equilibrium play in a thermometer?
A thermometer works by reaching thermal equilibrium with the object it measures.
- The temperature reading is accurate only when thermometer and object have same temperature.
- Thermal equilibrium ensures reliable temperature measurement.
10. Can thermal equilibrium exist between three or more bodies?
Yes, thermal equilibrium can exist among three or more bodies if all are at the same temperature and no net heat flows between any pair.
- This principle is explained by the zeroth law of thermodynamics.
11. What is meant by steady state and thermal equilibrium?
Steady state refers to a condition where the temperature at every point in a system does not change with time, while thermal equilibrium means uniform temperature throughout and no heat flow.
- Steady state can have local heat flows, but thermal equilibrium has none.
- Thermal equilibrium is a special case of steady state.
12. Why is thermal equilibrium necessary to define temperature?
Defining temperature relies on the concept of thermal equilibrium, as temperature represents the condition under which two bodies exchange no net heat.
- Without thermal equilibrium, temperature cannot be compared meaningfully between objects.
























