




Difference Between SN1 and SN2 Reactions (Tabular Comparison)
In JEE Main organic chemistry, understanding the SN1 and SN2 reactions is essential for mastering nucleophilic substitution mechanisms. These two pathways control the way reactants like alkyl halides are transformed, and knowing how to distinguish and predict them is a high-frequency exam skill. Questions expect students to apply rate law logic, identify intermediate formation, and determine stereochemistry. Vedantu’s expert-written notes below will help you build the intuition needed for exam MCQs and reasoning questions.
Both SN1 and SN2 reactions appear in JEE’s Organic Compounds Containing Halogens and General Principles of Organic Chemistry chapters. They’re also directly compared in reasoning problems based on steric effects, substrate structure, nucleophile strength, and solvent type. Let’s first build a foundational understanding of each mechanism, then examine their identification, differences, and applications with JEE-style examples and shortcuts.
Introduction to SN1 and SN2 Reactions in Organic Chemistry
SN1 and SN2 reactions are cornerstone nucleophilic substitution mechanisms typically seen with alkyl halides or similar substrates. In the SN1 reaction (Substitution Nucleophilic Unimolecular), the reaction proceeds via a two-step process with carbocation formation. SN2 (Substitution Nucleophilic Bimolecular) occurs via a direct, concerted one-step mechanism. The choice between the two routes depends on substrate structure, leaving group ability, nucleophile strength, and solvent properties. Key concepts like carbocation stability, transition state geometry, and inversion of configuration form the basis for most exam-level questions on this topic.
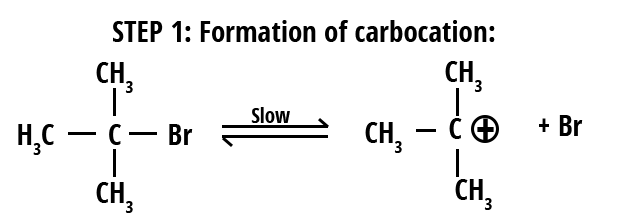
Mechanism of SN1 Reaction
The SN1 reaction follows a stepwise mechanism, making the process unique compared to SN2. Here’s how it proceeds:
- The leaving group (usually halide, -Br, -Cl, etc.) departs, forming a carbocation intermediate (slow, rate-determining step).
- The nucleophile attacks the planar carbocation to form the product (fast step), which can lead to racemization if the carbocation is chiral.
- In some cases, a deprotonation may follow if the nucleophile is neutral.
The rate law for SN1 is: Rate = k[substrate]. Only substrate concentration affects the rate since carbocation formation is the slowest step. Stability order for carbocation: tertiary > secondary > primary.
SN1 mechanisms are favored by polar protic solvents (like water, alcohols), which stabilize both the carbocation and leaving group, and by substrates that can easily form stable carbocations (tertiary alkyl halides, allylic, benzylic).
Formation of carbocation is the hallmark of SN1. In SN1, product stereochemistry is generally a racemic mixture due to nucleophile attack from either side.
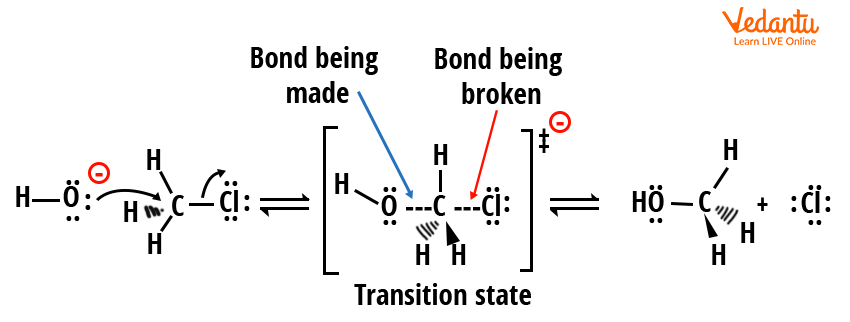
Mechanism of SN2 Reaction
The SN2 mechanism features a single concerted step—bond formation and bond breaking happen simultaneously:
- The nucleophile approaches the electrophilic carbon from the side opposite to the leaving group (backside attack).
- As the nucleophile forms a new bond, the leaving group departs, creating a transition state with partial bonds.
- Whole process occurs in one step; the carbon undergoes inversion of configuration (Walden inversion).
Rate law for SN2: Rate = k[substrate][nucleophile]. Both substrate and nucleophile concentrations control the reaction speed.
The SN2 pathway is favored for primary or methyl substrates, strong nucleophiles, and polar aprotic solvents (e.g., DMSO, acetone). Crowded (bulkier) substrates hinder the SN2 pathway due to sterics. Stereochemistry: only inversion is observed if the carbon is chiral.
The mechanism of SN2 reaction as seen in JEE is defined by inversion of configuration.
Difference Between SN1 and SN2 Reactions
For efficient exam revision and MCQ-solving, use this side-by-side comparison:
| Criteria | SN1 Reaction | SN2 Reaction |
|---|---|---|
| Rate Law | Rate = k[substrate] | Rate = k[substrate][nucleophile] |
| Number of Steps | Two-step (carbocation intermediate) | One-step (single concerted transition state) |
| Substrate Preference | Tertiary > Secondary > Primary | Methyl > Primary > Secondary |
| Nucleophile | Usually weak | Always strong |
| Solvent Effect | Favored by polar protic | Favored by polar aprotic |
| Stereochemistry | Racemization (mix) | Inversion only |
| Intermediate | Carbocation forms | No intermediate |
| Typical Example | Tert-butyl bromide + H2O | Methyl bromide + OH- |
The difference between SN1 and SN2 reactions can usually be remembered by focusing on the substrate, stereochemistry, and rate law.
How to Identify SN1 or SN2 Mechanism Quickly
In JEE Main and Advanced, you must identify the mechanism type from reactants and conditions. Use these rapid checks:
- Check the substrate: tertiary/benzylic/allylic = likely SN1; primary/methyl = likely SN2.
- Look at the nucleophile: strong and small (e.g., OH-, CN-) = SN2; weak, bulky, or neutral (e.g., H2O, ROH) = SN1.
- Assess solvent type: polar protic (water, alcohol)=SN1; polar aprotic (acetone, DMSO)=SN2.
- Note possible rearrangements; SN1 can show hydride/alkyl shifts via carbocation.
- Bulky leaving group or crowded carbon hinders SN2; promotes SN1.
- Stereochemistry clue: single product with inversion = SN2; mixture = SN1.
Example: On encountering "2-bromo-2-methylpropane in water," the tertiary substrate and protic solvent point clearly to SN1, with formation of a racemic mixture expected.
SN1 and SN2: JEE-Level Reaction Examples
Let’s break down two standard exam-type reactions—one for each mechanism:
- SN1 Example: (CH3)3CBr + H2O → (CH3)3COH + HBr. Here, the tertiary alkyl halide forms a stable carbocation and the water attacks as nucleophile, giving tert-butanol with racemization.
- SN2 Example: CH3Br + OH- → CH3OH + Br-. Strong nucleophile attacks methyl bromide directly; inversion is not visually detectable but still occurs.
- In advanced examples, look for rearrangement (SN1) or rate dependence on nucleophile (SN2).
Exam Practice: Short JEE MCQ Style Questions
- Which reacts faster via SN2: methyl chloride or tertiary butyl chloride? Answer: methyl chloride, less sterically hindered.
- Predict the product of 1-bromo-2-methylpropane + aqueous NaOH. Likely SN2 (primary carbon), so inversion at the site of attack.
- What favors SN1 in secondary alkyl halides? Use polar protic solvent, weak nucleophile, and heat.
Top Student Pitfalls and Exam Tips
- Don’t assume all substitutions are SN2—reactivity order and solvent matter.
- Watch for rearrangements: if the intermediate shifts, it is definitely SN1.
- For optical isomers, only SN2 gives 100% inversion; SN1 gives mixture (racemization).
- Polar aprotic solvents (like acetone, DMF) speed up SN2 by boosting nucleophilicity.
- Rate of SN1 does not depend on nucleophile strength—carbocation forms first.
- Always redraw chiral centers to check stereochemistry after the reaction.
Key Linked Concepts Worth Reviewing
- Organic Compounds Containing Halogens: Key Questions
- Nucleophilic Substitution Mechanism Basics
- Nucleophilic Substitution Types
- Difference Between Electrophiles and Nucleophiles
- Carbocation Rearrangement
- Reaction Intermediates and Stability
- General Principles of Organic Chemistry
- Alkyl Halide Practice Test
- E1 and Related Reactions
For faster revision, download Vedantu’s concise SN1 vs SN2 tables or attempt mock JEE Chemistry tests online. Mastering these mechanisms will also help you answer assertion-reasoning and ‘mechanism-based product prediction’ MCQs commonly asked in JEE Main and JEE Advanced.
FAQs on SN1 and SN2 Reactions Explained: Mechanisms, Differences, and Examples
1. What is the difference between SN1 and SN2 reactions?
SN1 and SN2 reactions are two different types of nucleophilic substitution mechanisms in organic chemistry.
Key differences include:
- SN1: Two-step process, forms carbocation intermediate, generally seen in tertiary alkyl halides, leads to racemization.
- SN2: One-step, concerted mechanism with a transition state, occurs with primary alkyl halides, results in inversion of configuration.
- SN1 rate: Depends only on substrate; SN2 rate: Depends on both substrate and nucleophile.
2. How do you identify if a reaction follows the SN1 or SN2 mechanism?
You can identify whether a reaction is SN1 or SN2 by examining the substrate, nucleophile, solvent, and reaction conditions.
Tips to identify:
- Tertiary alkyl halide + weak nucleophile + polar protic solvent → usually SN1.
- Primary/ methyl halide + strong nucleophile + polar aprotic solvent → usually SN2.
- Check for stereochemistry: inversion (SN2), racemization (SN1).
3. What type of substrate favors SN1 reactions?
SN1 reactions are favored by substrates that can form stable carbocations.
Substrates that commonly undergo SN1:
- Tertiary alkyl halides (most favorable due to carbocation stability)
- Allylic and benzylic halides (due to resonance stabilization of the carbocation)
- Secondary alkyl halides (under certain conditions)
4. Why is inversion of configuration observed in SN2 reactions?
In SN2 reactions, inversion of configuration happens because the nucleophile attacks from the side opposite to the leaving group (backside attack).
This causes the stereochemistry to flip, also known as the Walden inversion:
- Only one transition state is formed
- Occurs mainly in chiral centers
- Results in 100% inversion of configuration
5. Can you give examples of SN1 and SN2 reactions asked in JEE?
Yes, JEE exams commonly ask the following examples:
SN1 Example: Hydrolysis of tert-butyl bromide ((CH3)3CBr) in aqueous solution gives tert-butyl alcohol.
SN2 Example: Reaction of methyl bromide (CH3Br) with hydroxide ion forms methanol.
Both mechanisms test your understanding of substrate, nucleophile, and stereochemistry.
6. Does SN2 ever occur in tertiary alkyl halides?
SN2 reactions very rarely occur with tertiary alkyl halides due to strong steric hindrance.
- Bulky groups prevent nucleophile's backside attack
- SN1 is strongly favored for tertiary substrates
- Almost all nucleophilic substitutions for such substrates are SN1
7. Are all nucleophilic substitutions either SN1 or SN2?
Not all nucleophilic substitutions are strictly SN1 or SN2 — some reactions display mixed or borderline mechanisms.
- Some reactions proceed via SNi or SNAr mechanisms
- Secondary substrates may show a blend of SN1/SN2 features
- Exam questions may test recognition of such exceptions
8. How do polar protic and aprotic solvents affect SN1/SN2?
Solvent plays a key role in the rate and type of substitution reaction.
- Polar protic solvents (e.g., water, alcohol): Stabilize carbocations, thus favor SN1 mechanism.
- Polar aprotic solvents (e.g., DMSO, acetone): Do not stabilize carbocations; increase nucleophile strength, thus favor SN2 reactions.
9. Is retention of configuration possible in SN1 reactions?
Yes, retention of configuration can occur in SN1 reactions if the nucleophile attacks from both sides of the planar carbocation intermediate.
- Usually results in a racemic mixture (both inversion and retention products).
- In some cases, slight preference if steric hindrance exists.
10. What are the key features of SN1 and SN2 reaction mechanisms?
The key features of SN1 and SN2 mechanisms include the number of steps, intermediates, and reaction rates.
- SN1: Two steps, involves carbocation, unimolecular rate-determining step, possible rearrangements, racemization.
- SN2: Single-step, concerted, bimolecular, backside attack, inversion of configuration, no rearrangement.
11. Can you list factors affecting the rate of SN1 and SN2 reactions?
Several factors impact the rate of SN1 and SN2 reactions:
- SN1: Stability of carbocation, nature of the substrate, quality of leaving group, solvent polarity.
- SN2: Strength of nucleophile, steric hindrance, substrate structure, solvent (aprotic).
12. What is meant by racemization in SN1 reactions?
Racemization in SN1 reactions occurs because the nucleophile can attack the carbocation intermediate from either side, resulting in both enantiomers.
- Forms a racemic mixture if original carbon is chiral
- Leads to loss of optical activity























