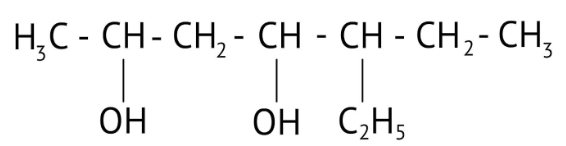
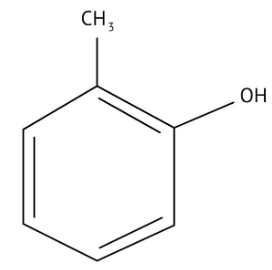
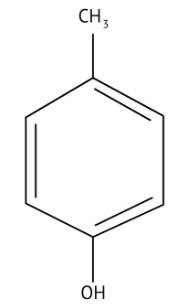
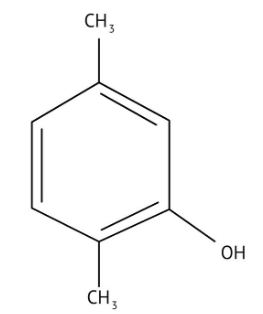
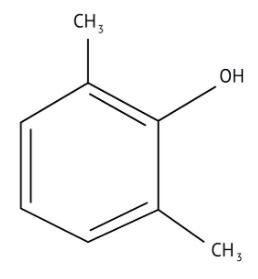
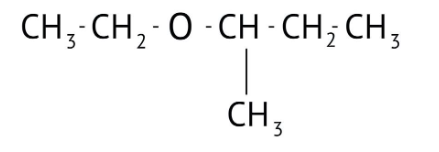
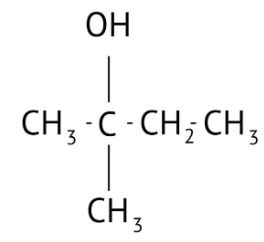
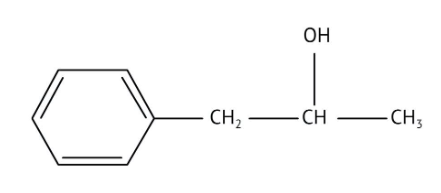
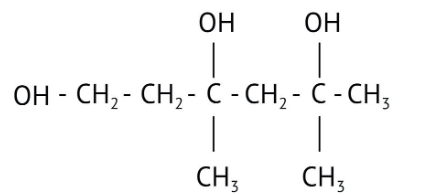
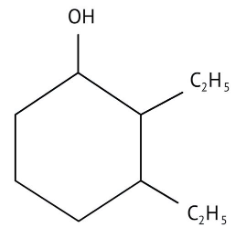
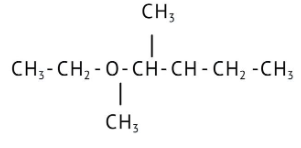
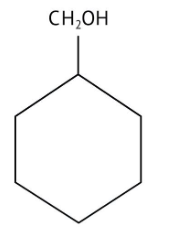
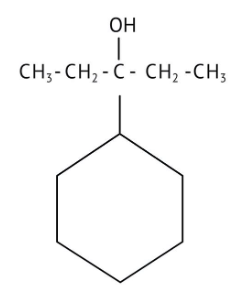
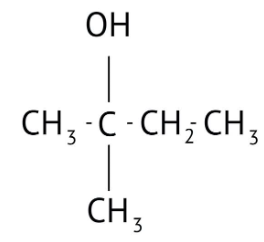
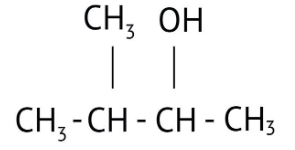
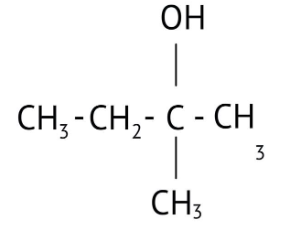
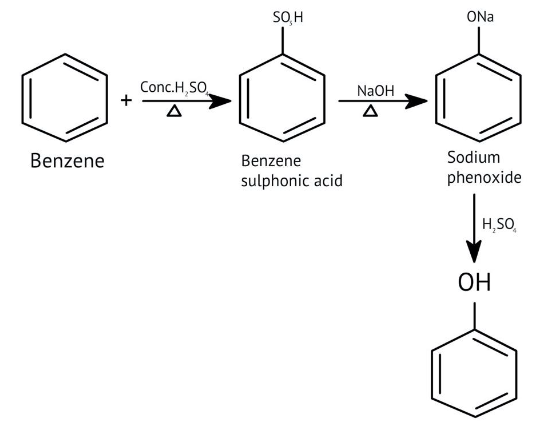
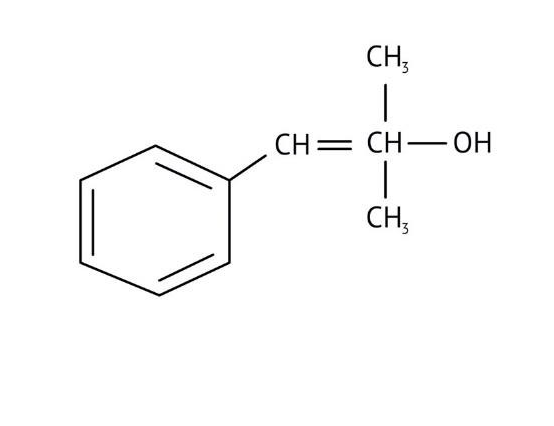
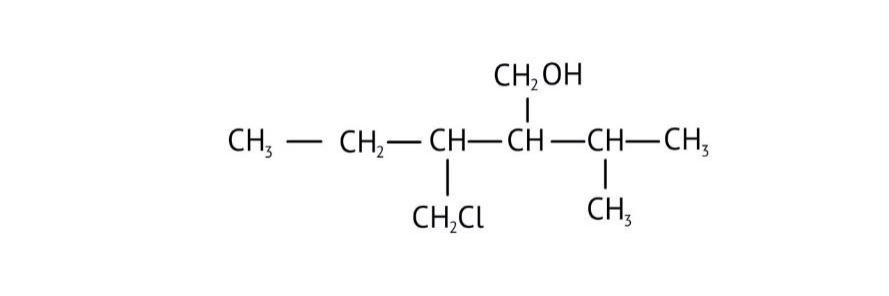
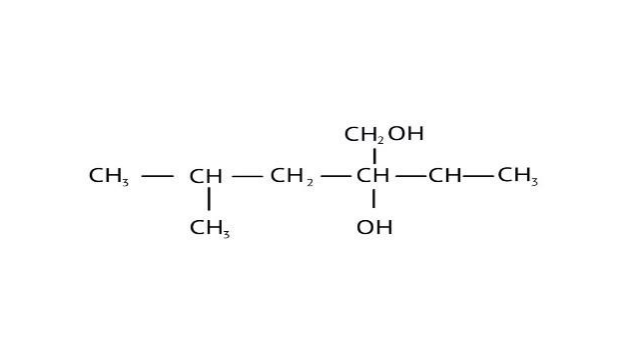
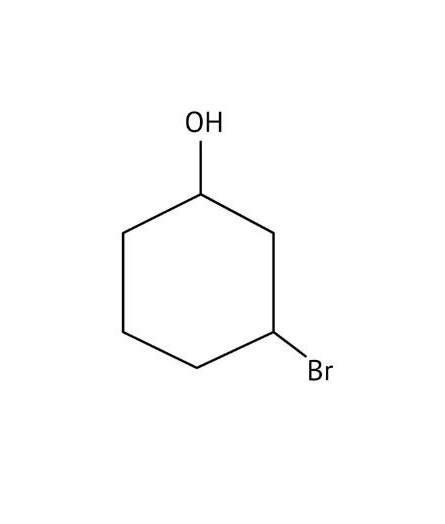
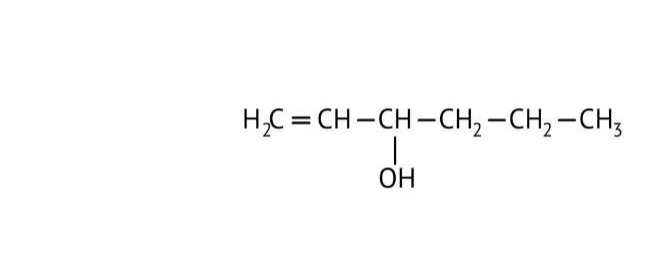
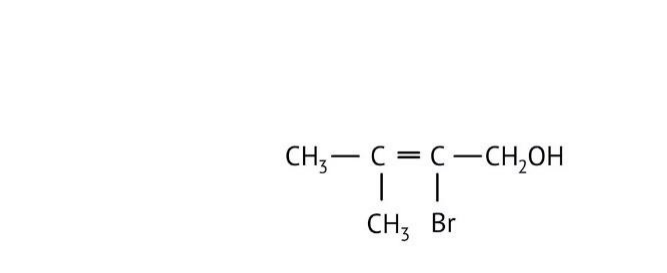
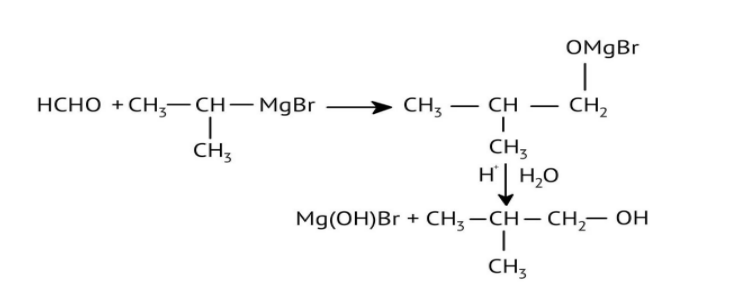
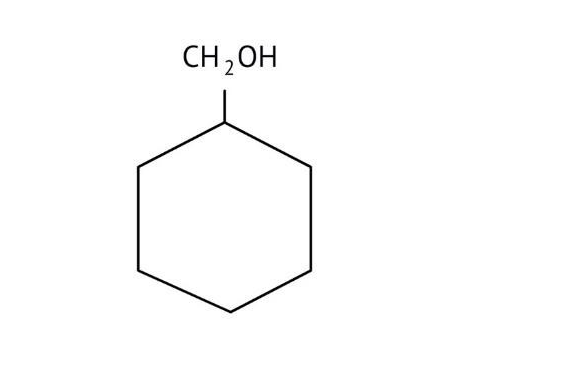
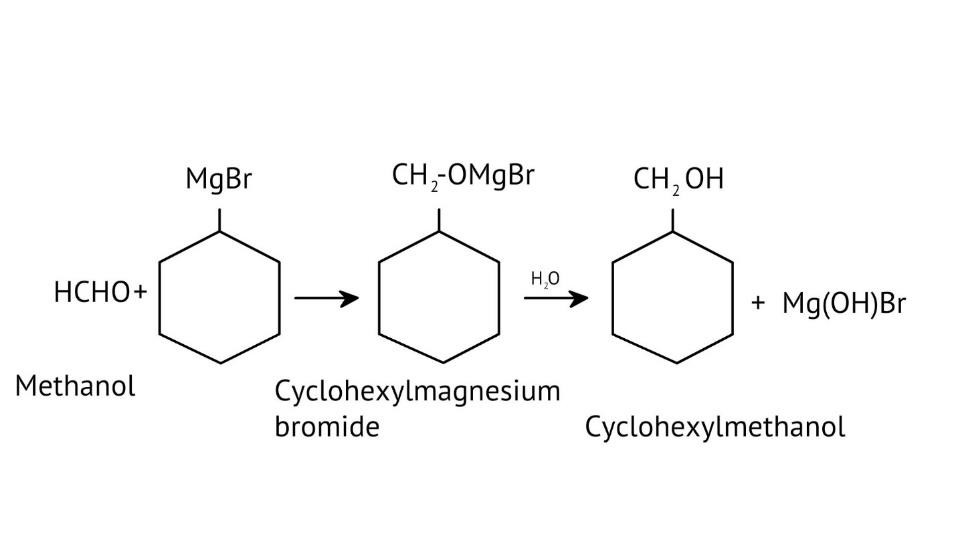
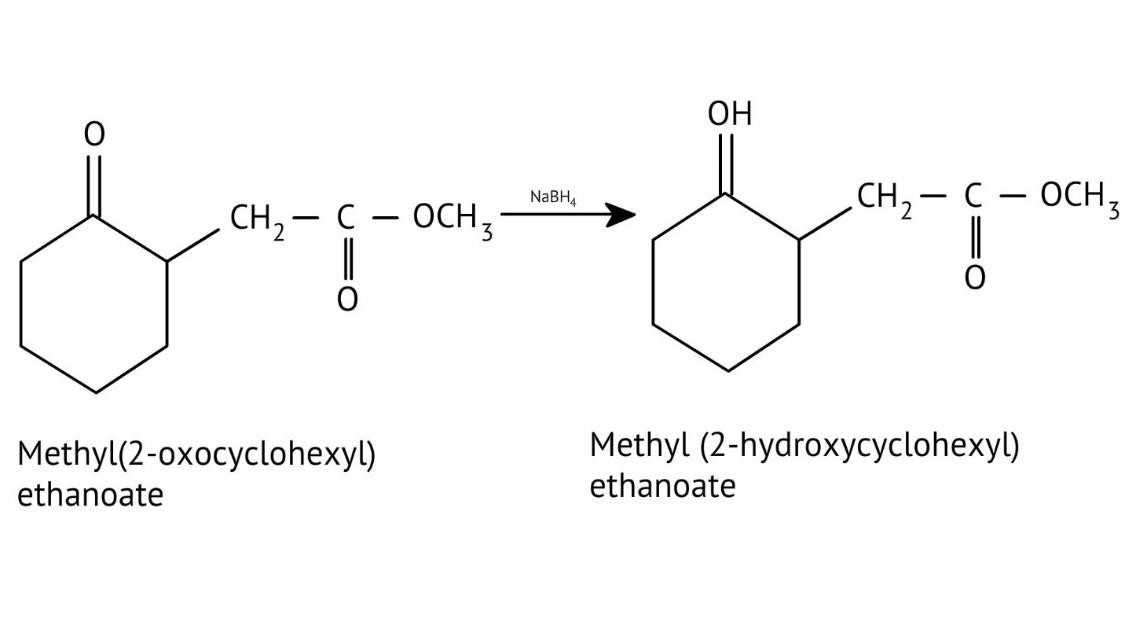
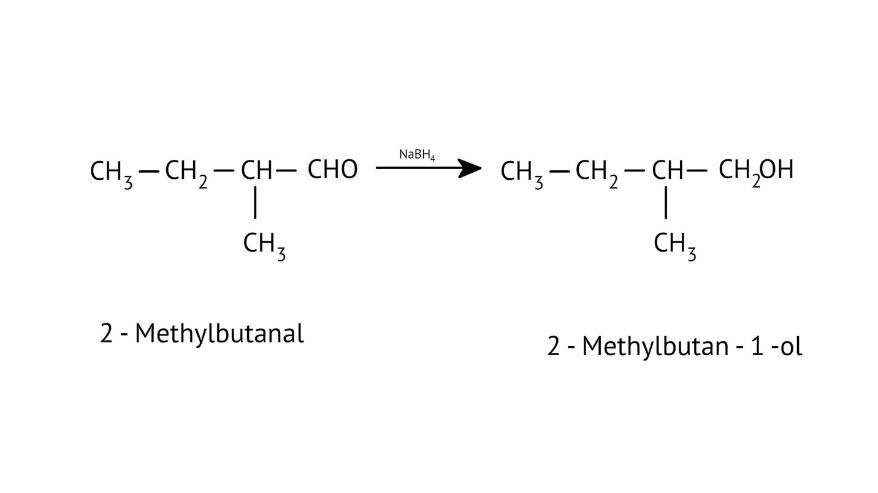
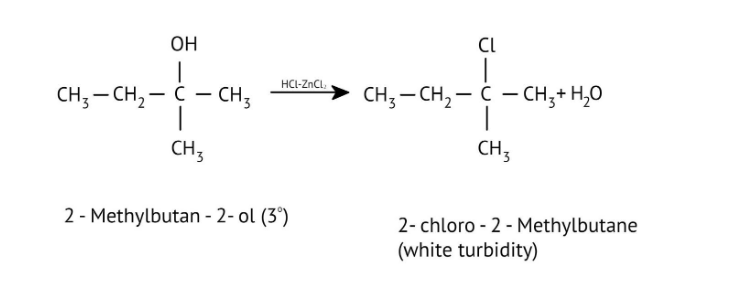
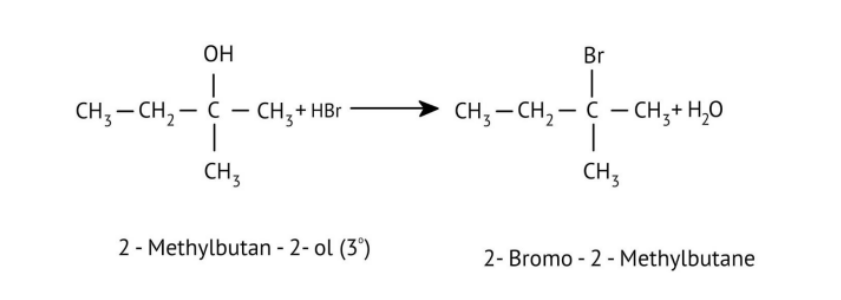
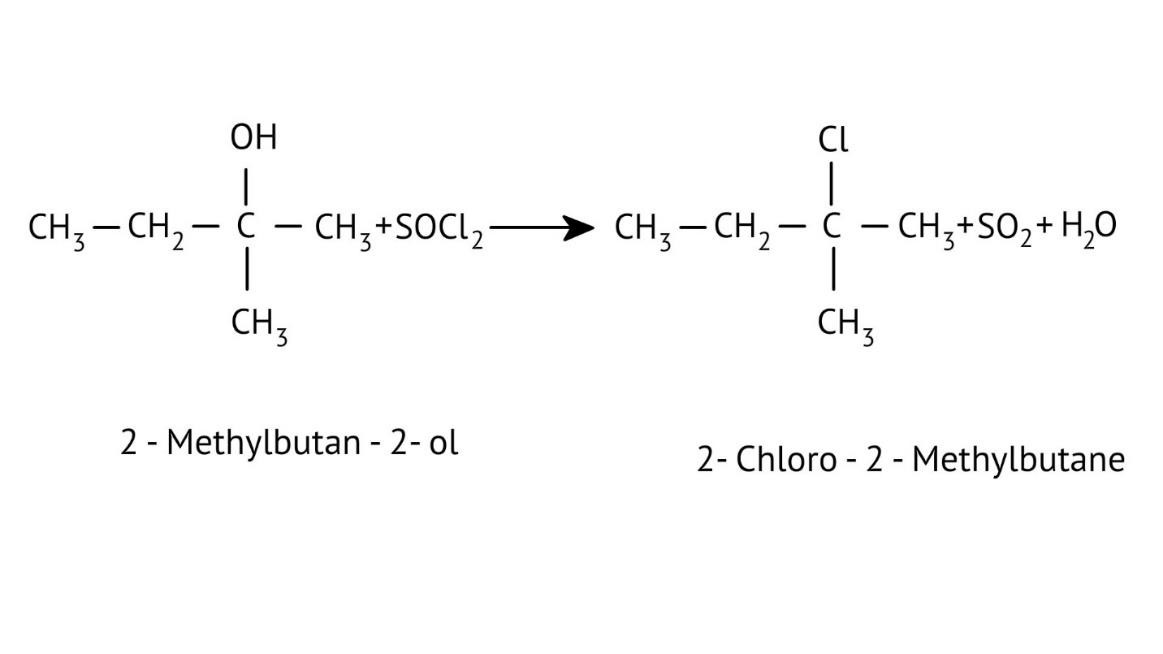
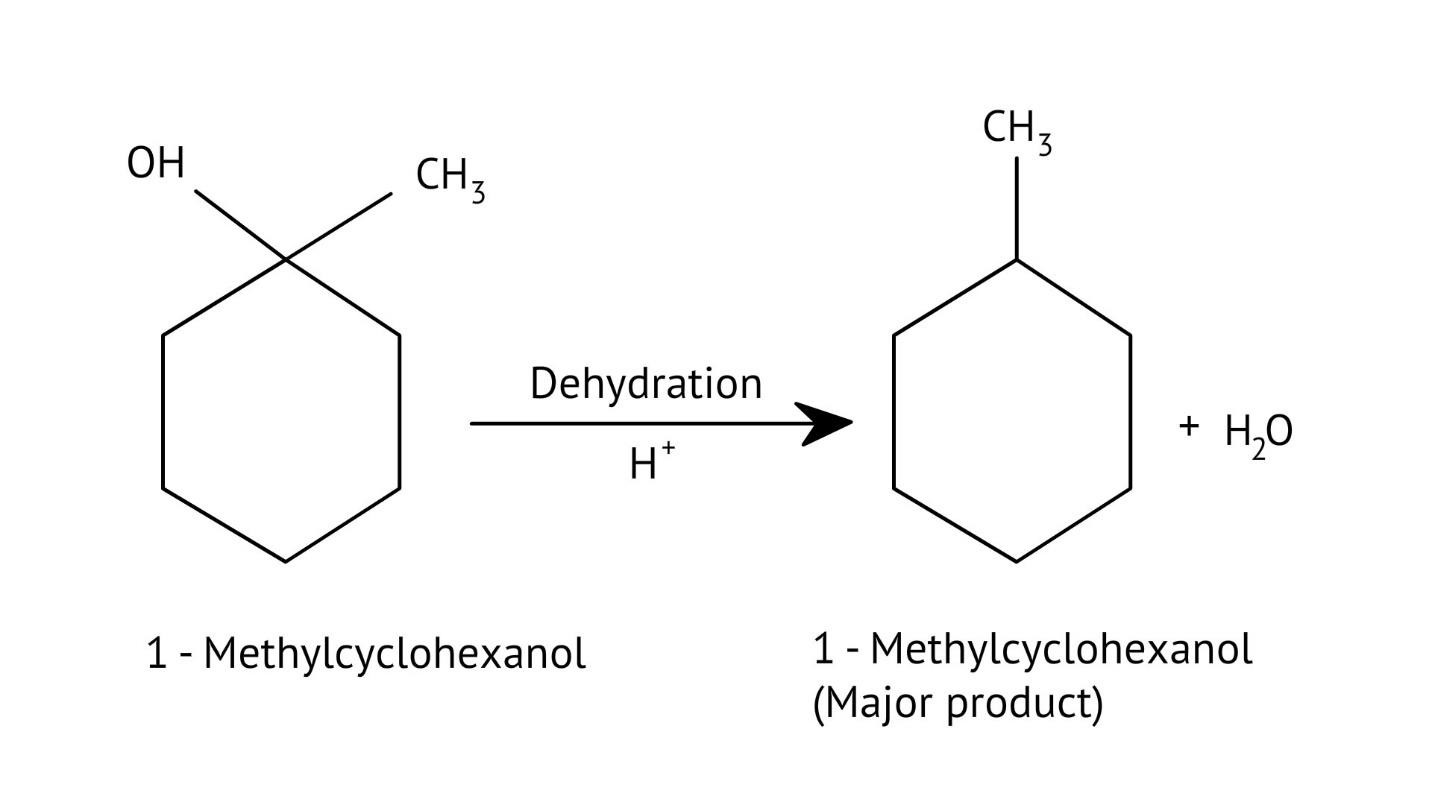
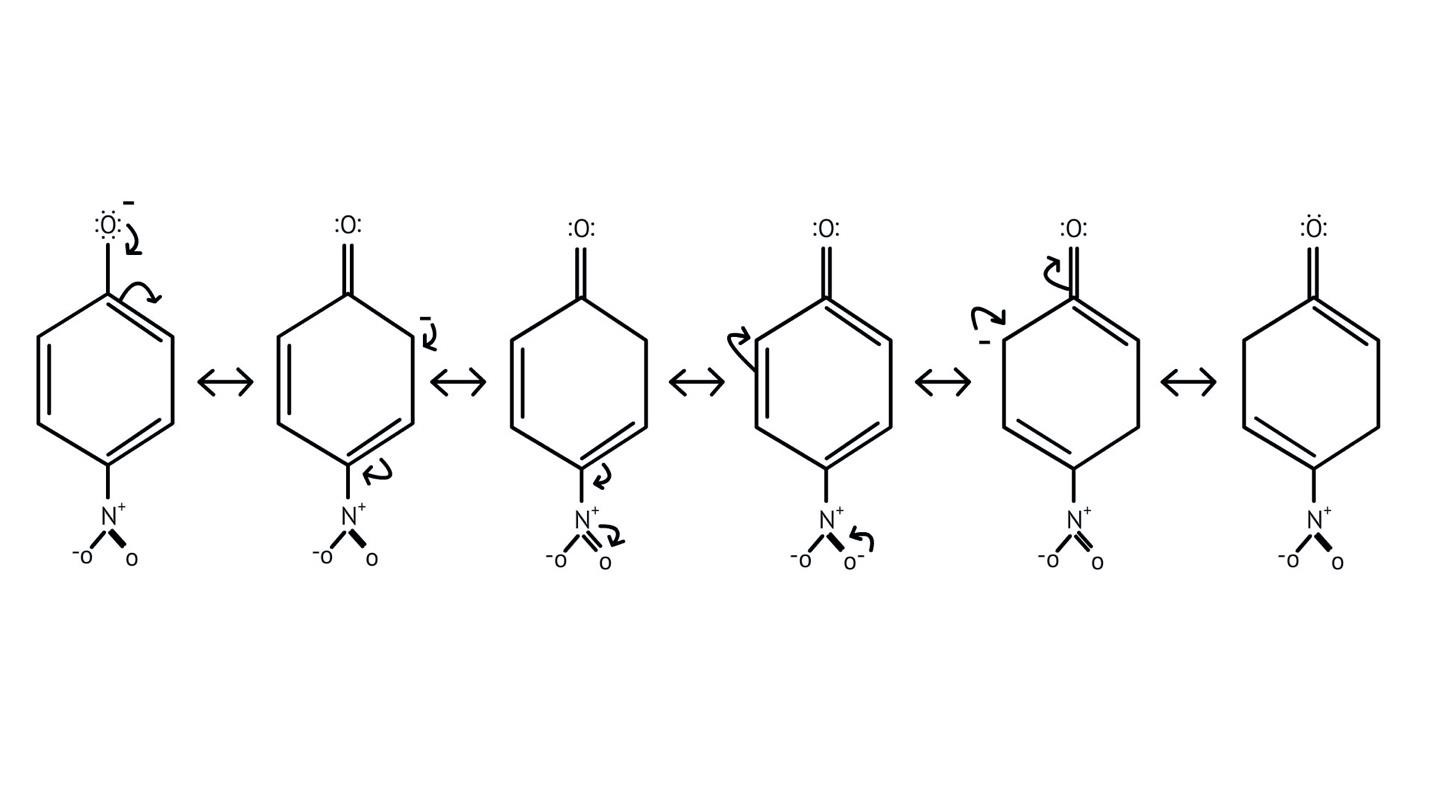
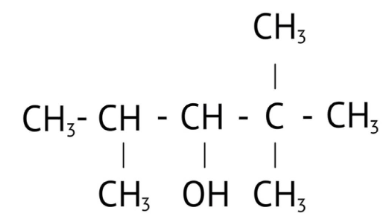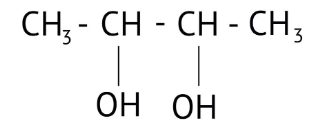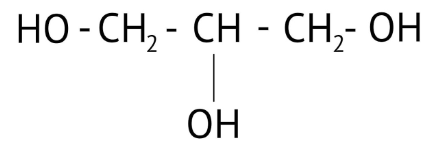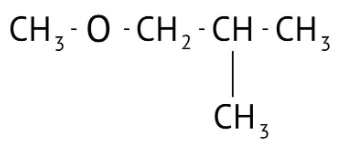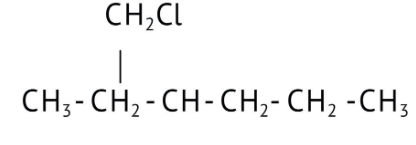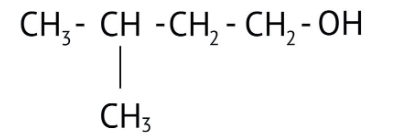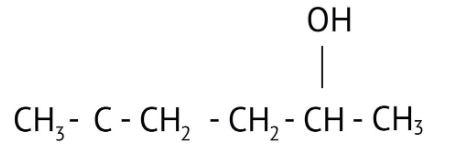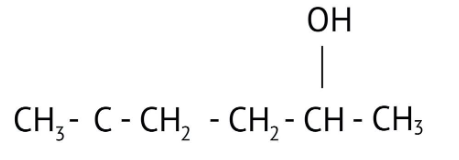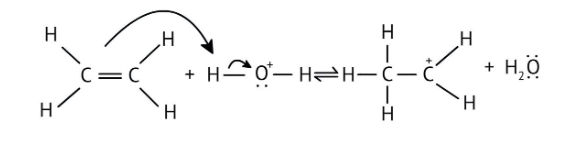How Can I Access Class 12 Chemistry Chapter 7 NCERT Solutions for Alcohol Phenol and Ether Questions and Answers for Exam Preparation?
FAQs on NCERT Solutions For Class 12 Chemistry Chapter 7 Alcohol Phenol And Ether - 2025-26
1. How do the NCERT Solutions for Class 12 Chemistry Chapter 7 help in preparing for the 2025-26 board exams?
The solutions provide detailed, step-by-step answers to all in-text and exercise questions, aligning perfectly with the latest CBSE 2025-26 syllabus. They help students master IUPAC nomenclature, reaction mechanisms, and named reactions like Kolbe's and Reimer-Tiemann, ensuring a strong foundation for exam questions.
2. Do these NCERT Solutions cover both the in-text and end-of-chapter exercise questions for Alcohols, Phenols and Ethers?
Yes, the NCERT Solutions provide comprehensive, stepwise answers for all questions, including the in-text examples and the end-of-chapter exercises. This ensures complete coverage of the concepts and problem types presented in the NCERT textbook.
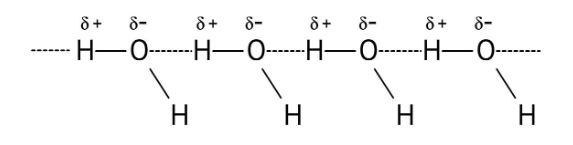
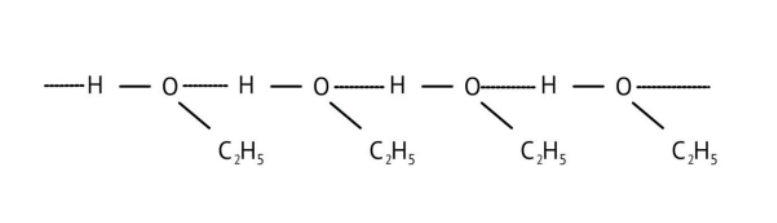
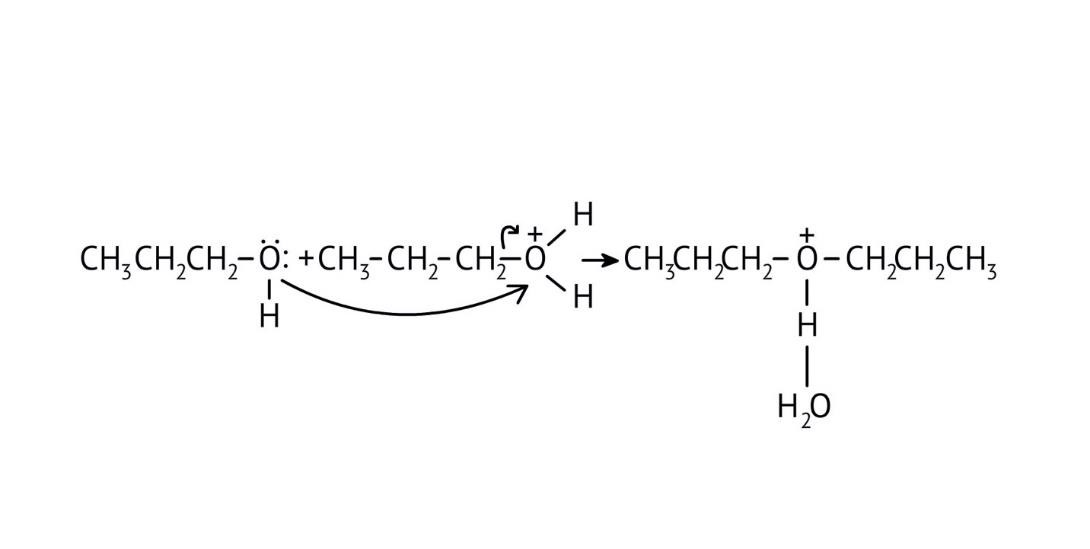
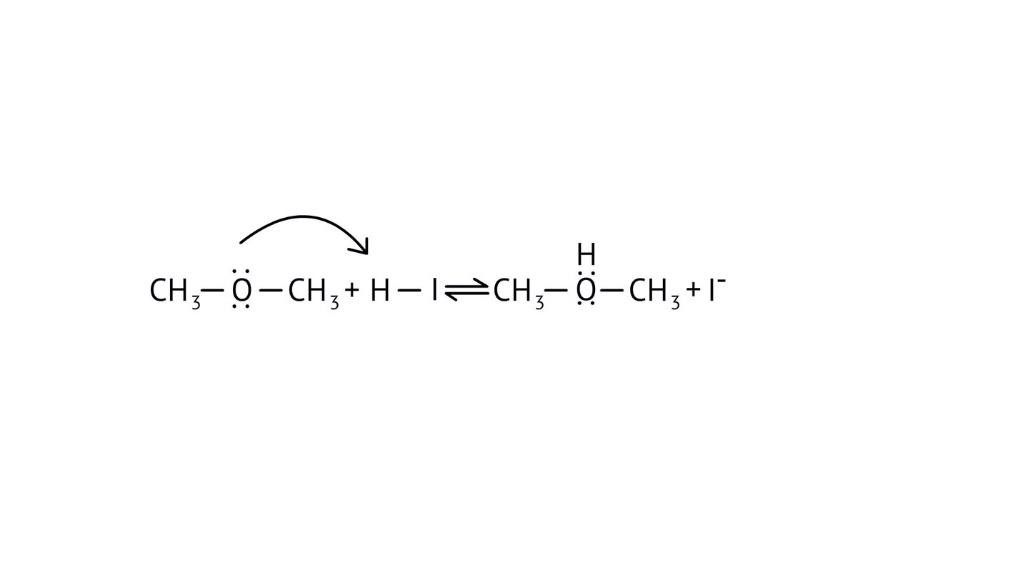
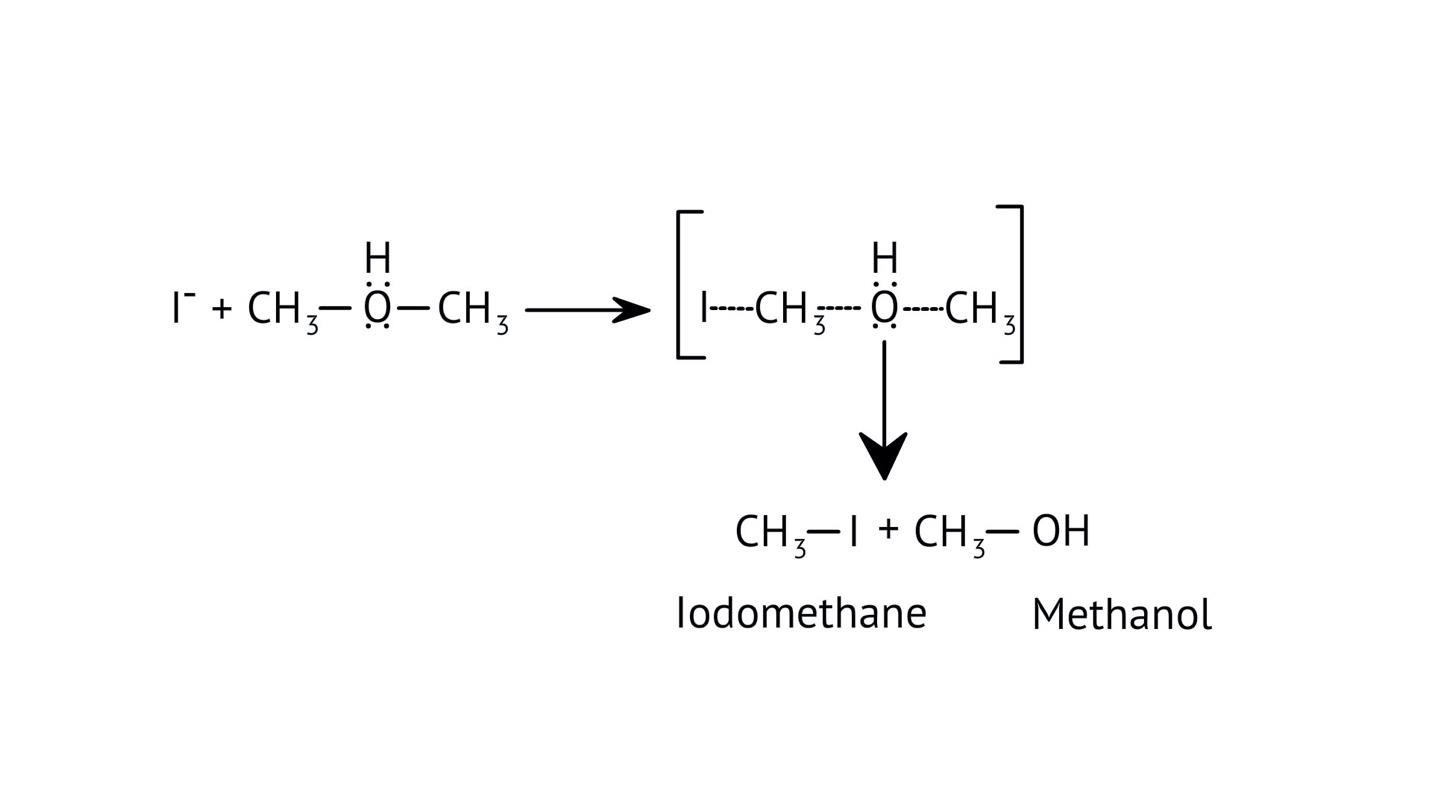
3. How do the NCERT Solutions explain the significant difference in boiling points between alcohols and ethers of comparable molecular mass?
The solutions explain that alcohols have much higher boiling points due to the presence of intermolecular hydrogen bonding between their -OH groups. Ethers, lacking this strong force, have weaker dipole-dipole interactions and thus lower boiling points. The solutions illustrate this principle clearly to solve conceptual questions.
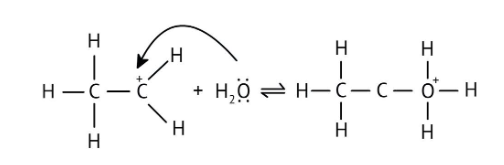
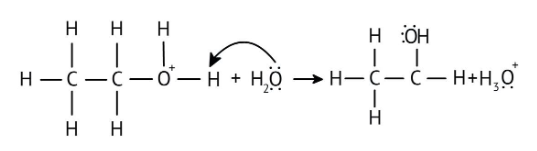
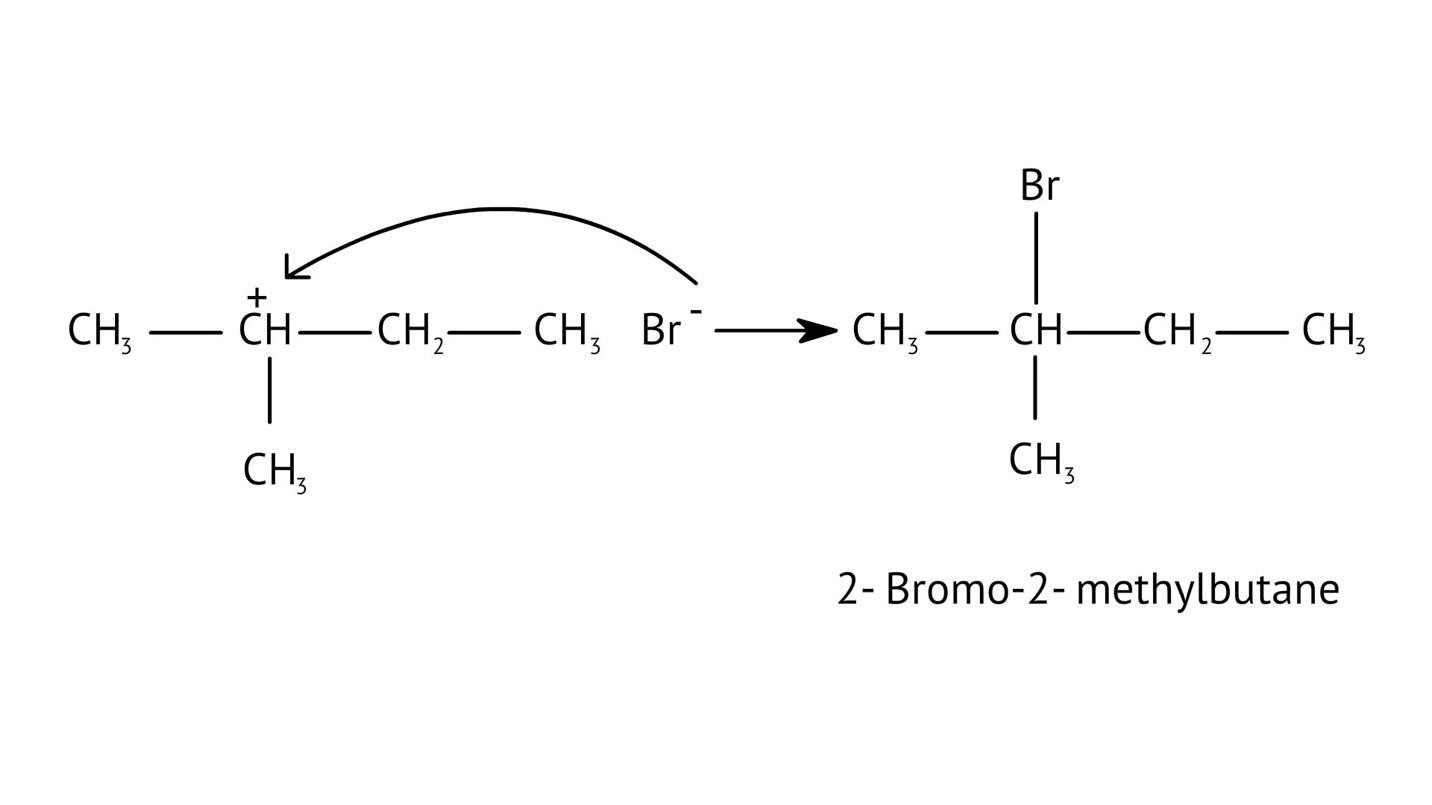
4. What is the step-by-step method recommended in the NCERT Solutions for solving reaction mechanism questions?
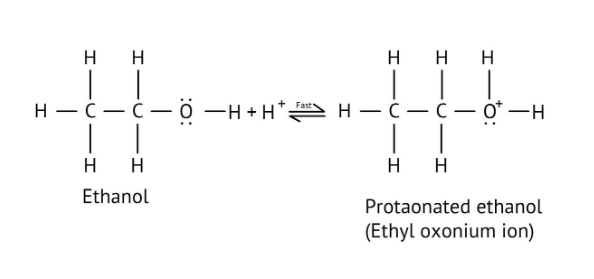
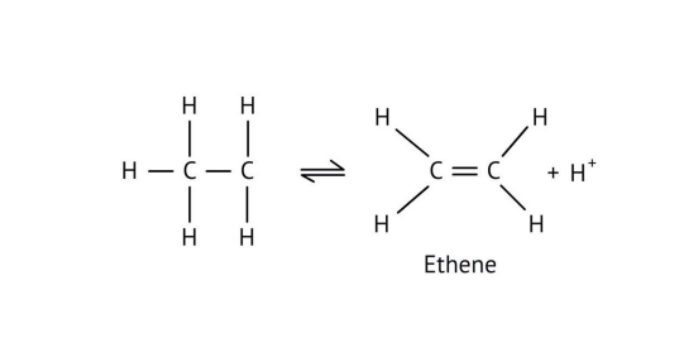
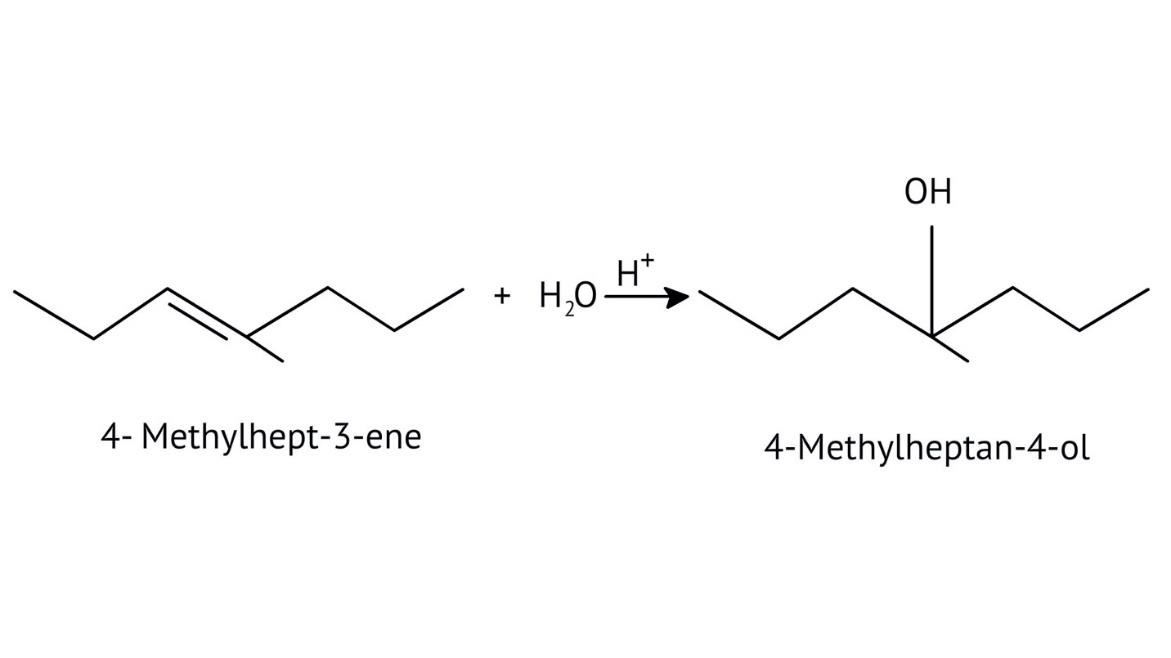
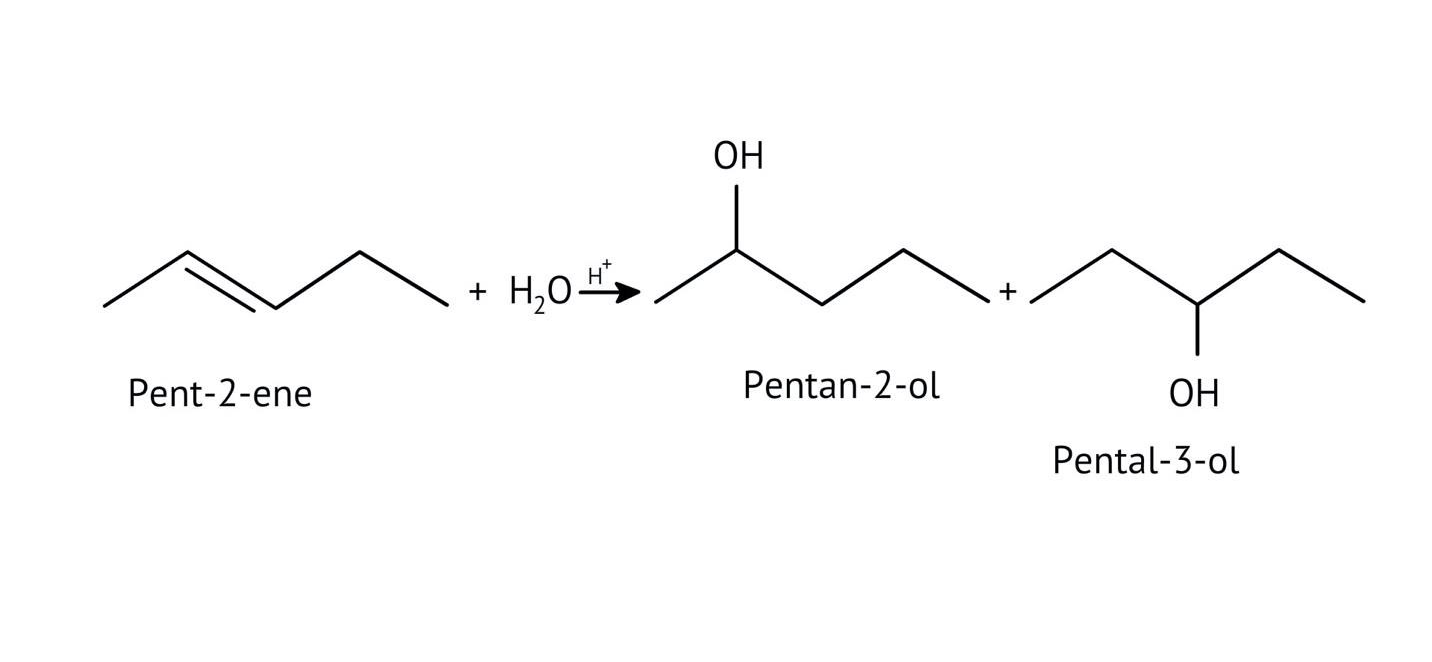
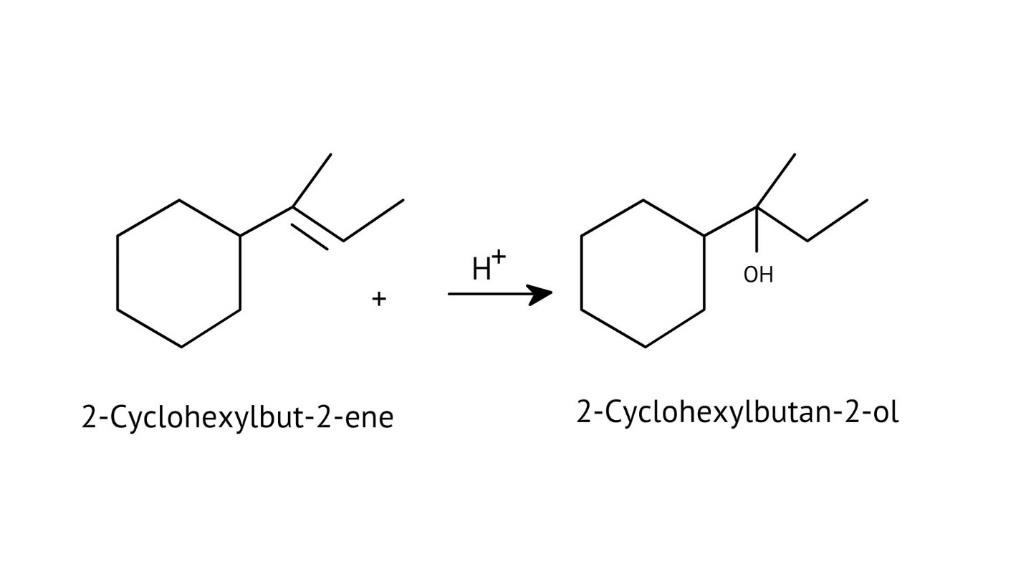
The solutions demonstrate a clear, multi-step method for mechanisms like the hydration of alkenes or dehydration of alcohols:
- Step 1: Protonation of the reactant (e.g., alcohol or alkene).
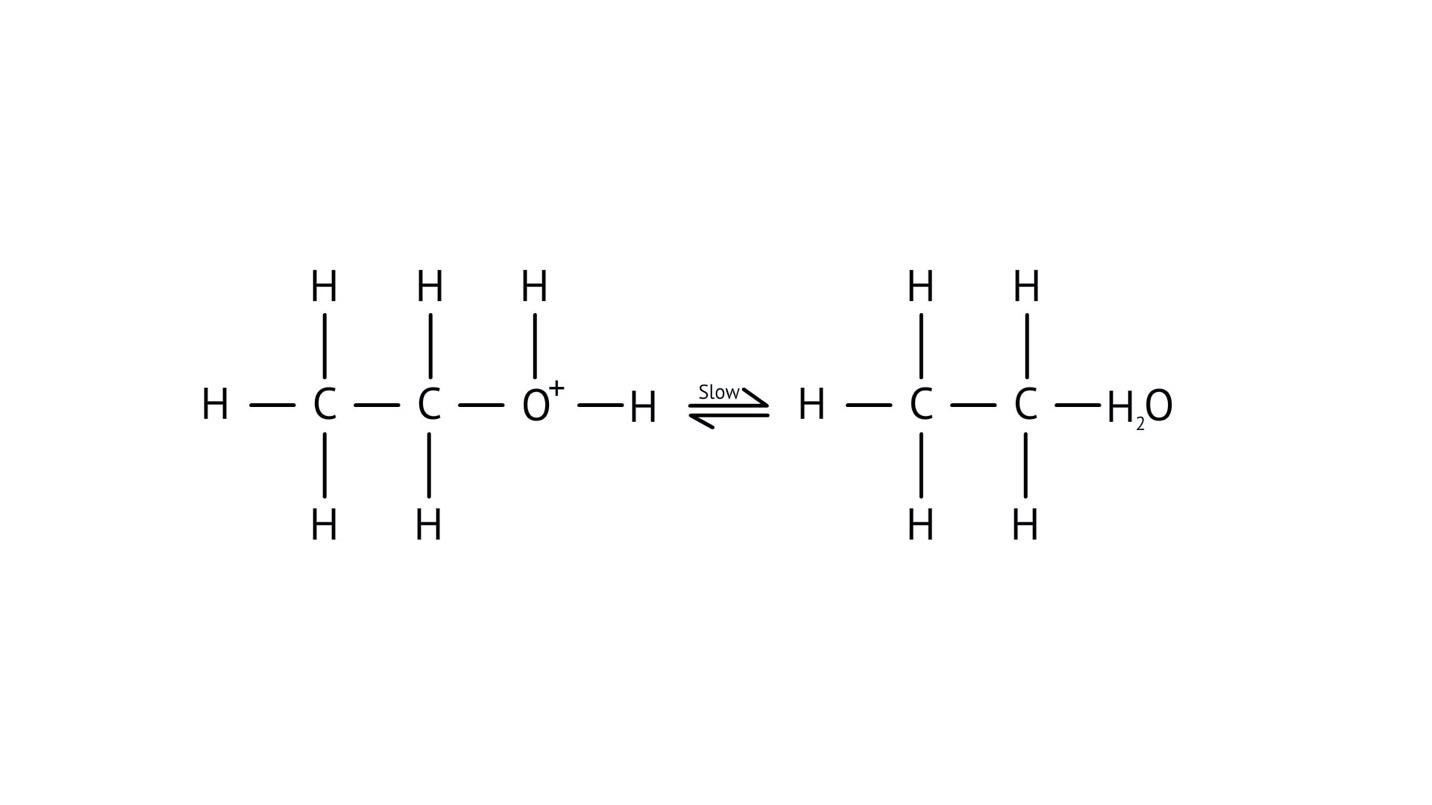
- Step 2: Formation of a carbocation intermediate.
- Step 3: Nucleophilic attack or rearrangement.
- Step 4: Deprotonation to yield the final product.
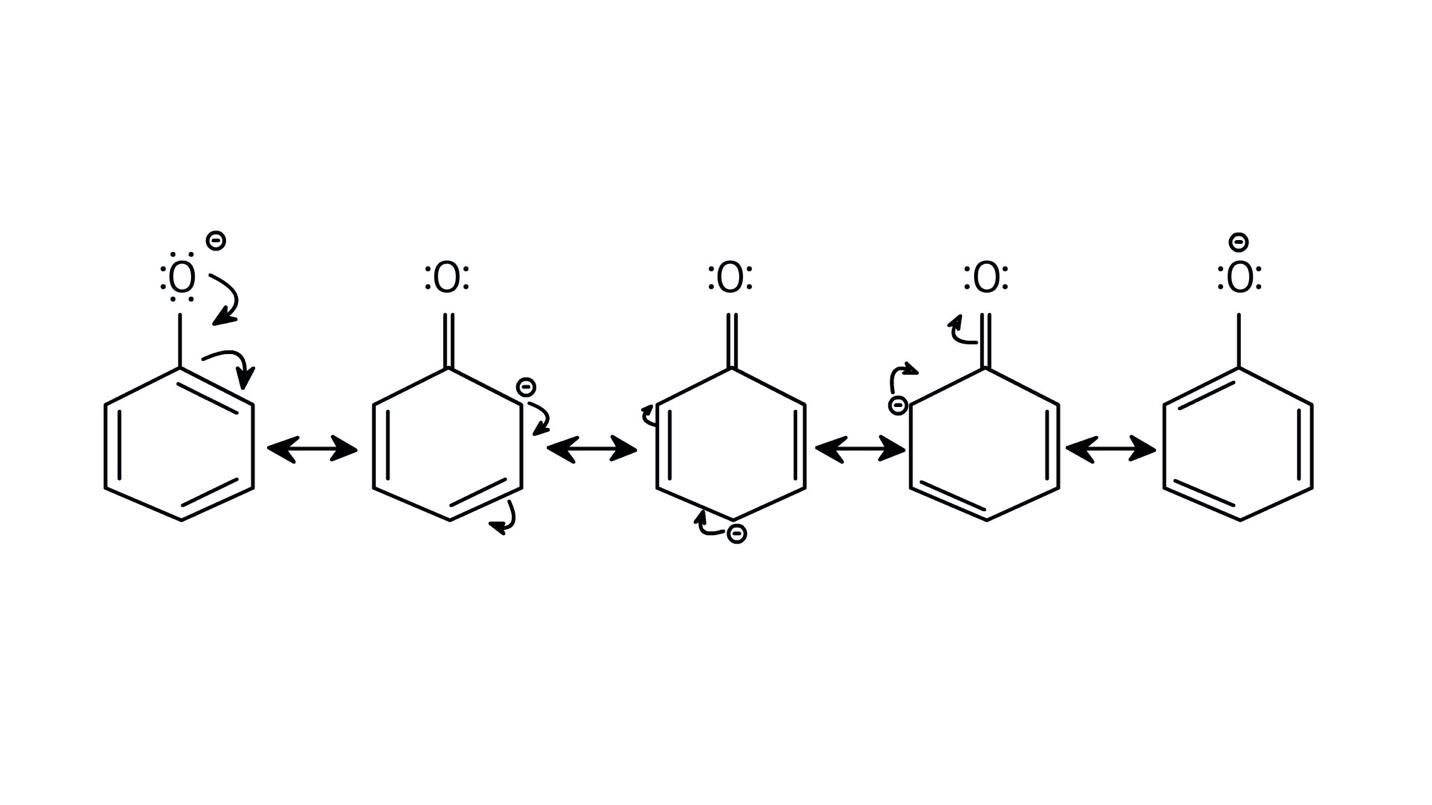
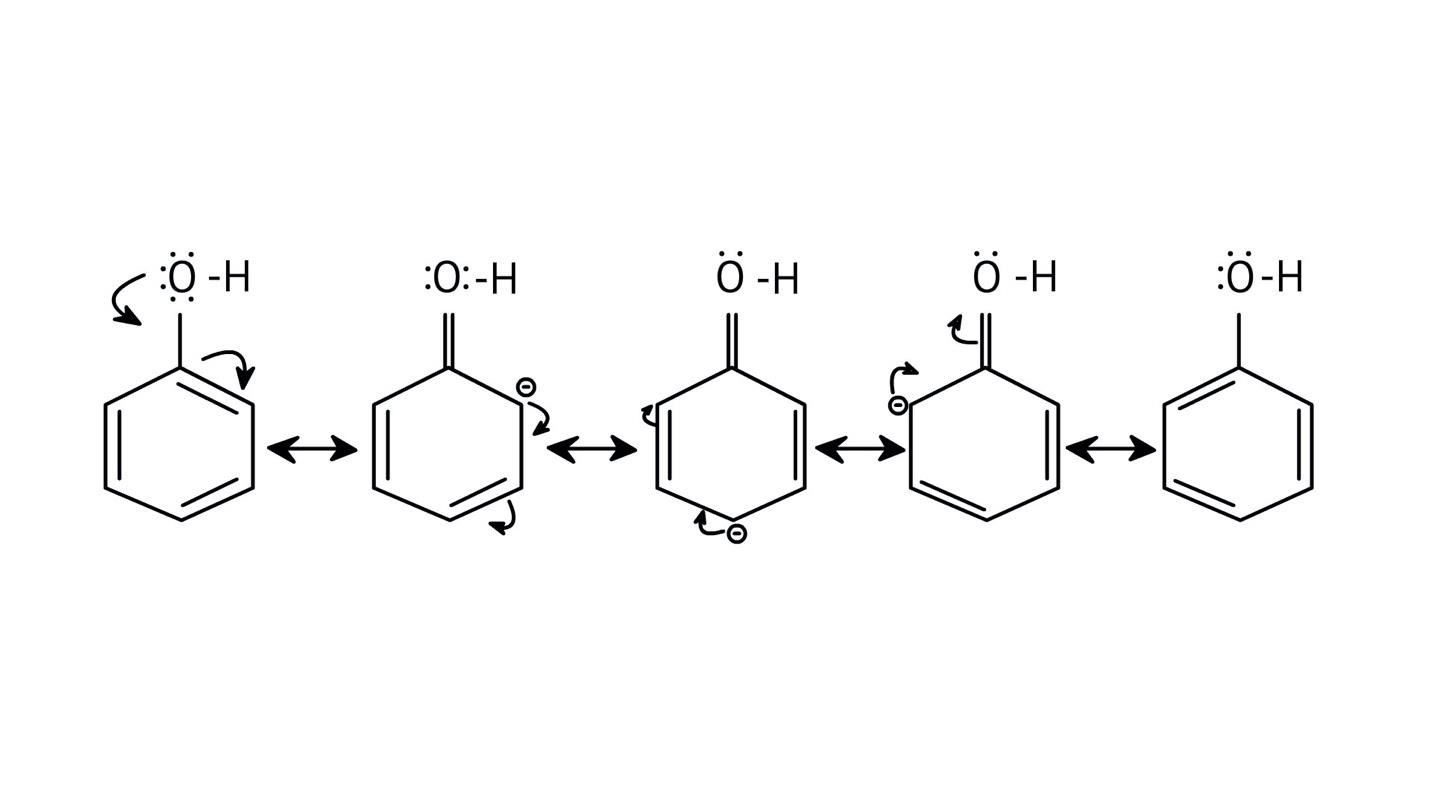
5. How do the solutions clarify why phenols are more acidic than alcohols?
The NCERT Solutions clarify that phenols are more acidic because the resulting phenoxide ion is highly stabilised by resonance, delocalising the negative charge over the benzene ring. In contrast, the alkoxide ion from an alcohol has its charge localised on the oxygen atom, making it less stable and less likely to form.
6. What common mistakes do the detailed solutions for Chapter 7 help students avoid in exams?
These solutions help students avoid common errors such as:
- Incorrectly applying Markovnikov's rule in hydration reactions.
- Confusing the products of oxidation for primary, secondary, and tertiary alcohols.
- Forgetting that Williamson Synthesis follows an SN2 pathway and fails with tertiary alkyl halides, which favour elimination.
- Misidentifying the major product in electrophilic substitution of phenols.
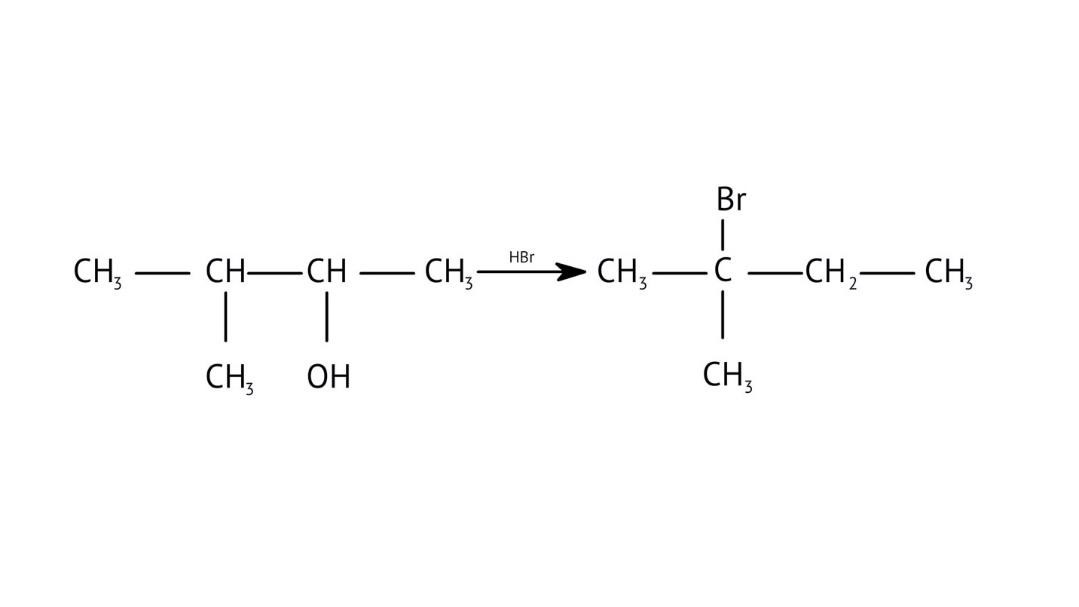
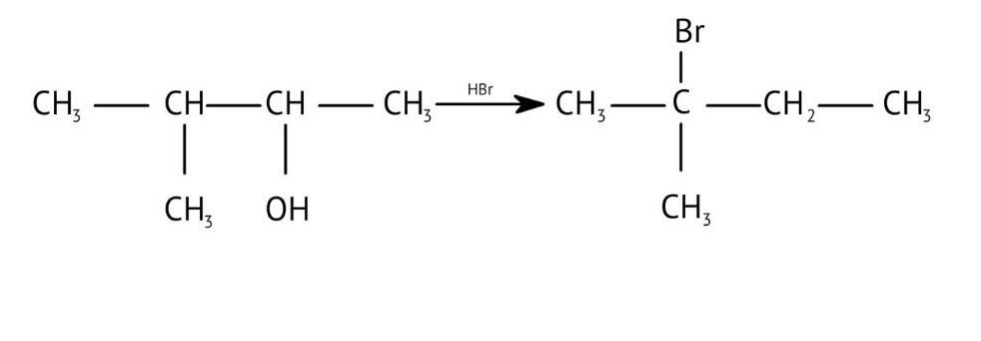
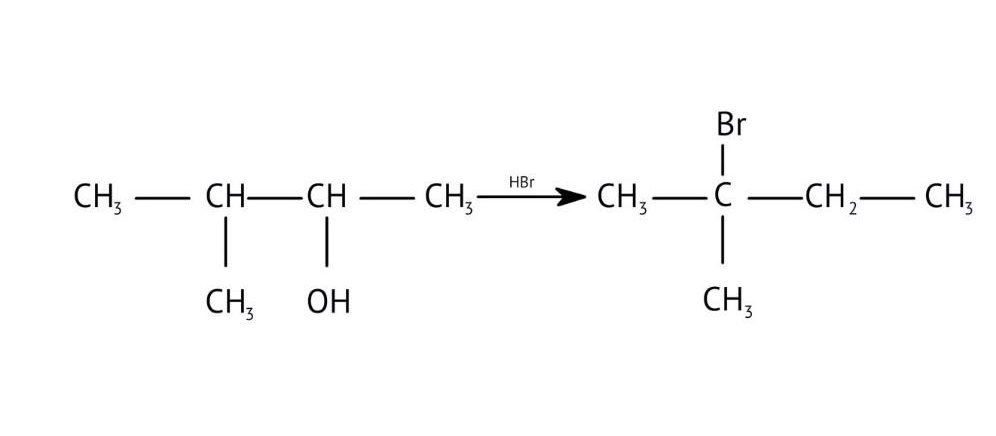
7. How are primary, secondary, and tertiary alcohols distinguished using the Lucas Test, as explained in the NCERT Solutions?
The NCERT Solutions explain the Lucas Test (using conc. HCl and ZnCl₂) as follows:
- Tertiary alcohols: React immediately to form a cloudy solution (turbidity).
- Secondary alcohols: Form turbidity within 5-10 minutes.
- Primary alcohols: Show no reaction at room temperature; turbidity appears only on heating.
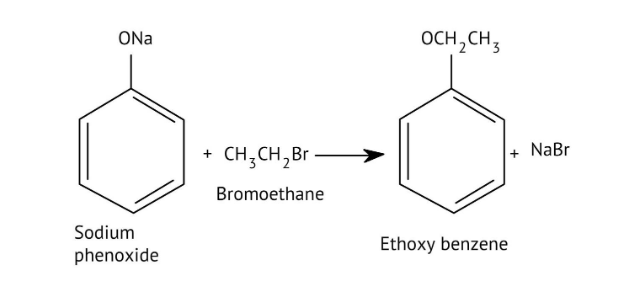
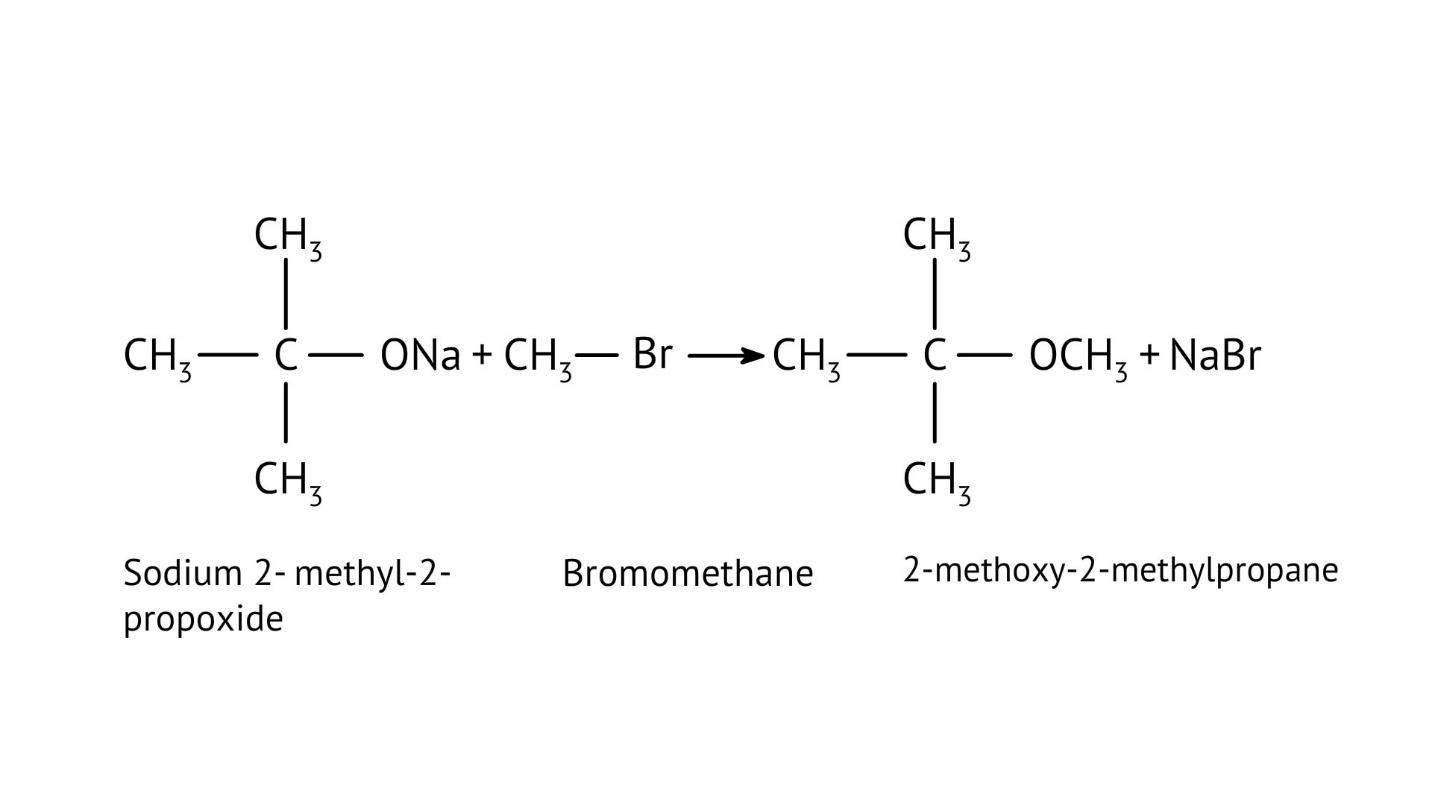
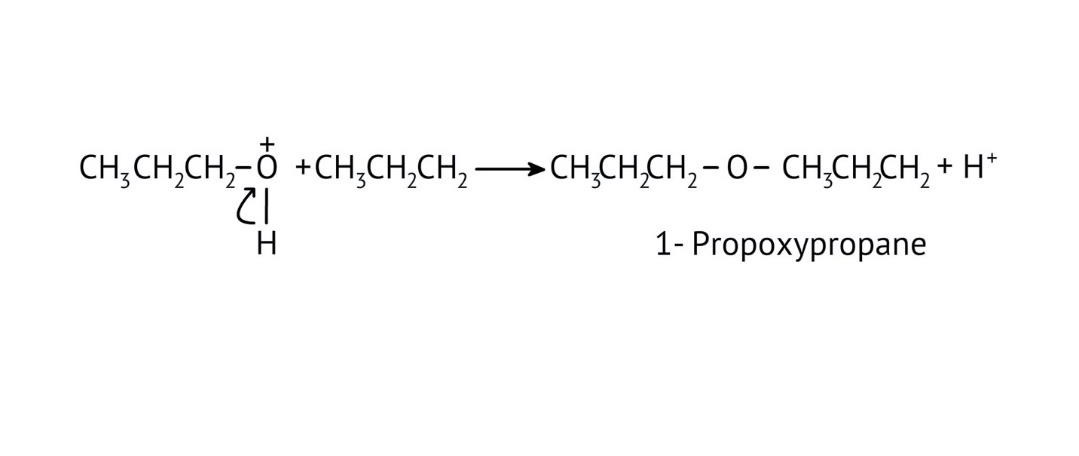
8. How do the NCERT Solutions explain the mechanism and limitations of Williamson Ether Synthesis?
The solutions explain Williamson Synthesis as an SN2 reaction between a sodium alkoxide and a primary alkyl halide. A key limitation highlighted is that if a secondary or tertiary alkyl halide is used, an elimination reaction occurs, producing an alkene instead of the desired ether, due to the alkoxide also acting as a strong base.
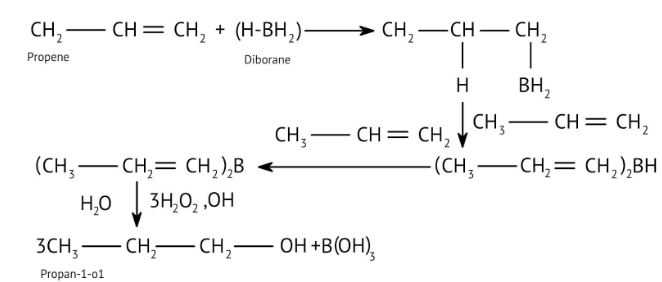
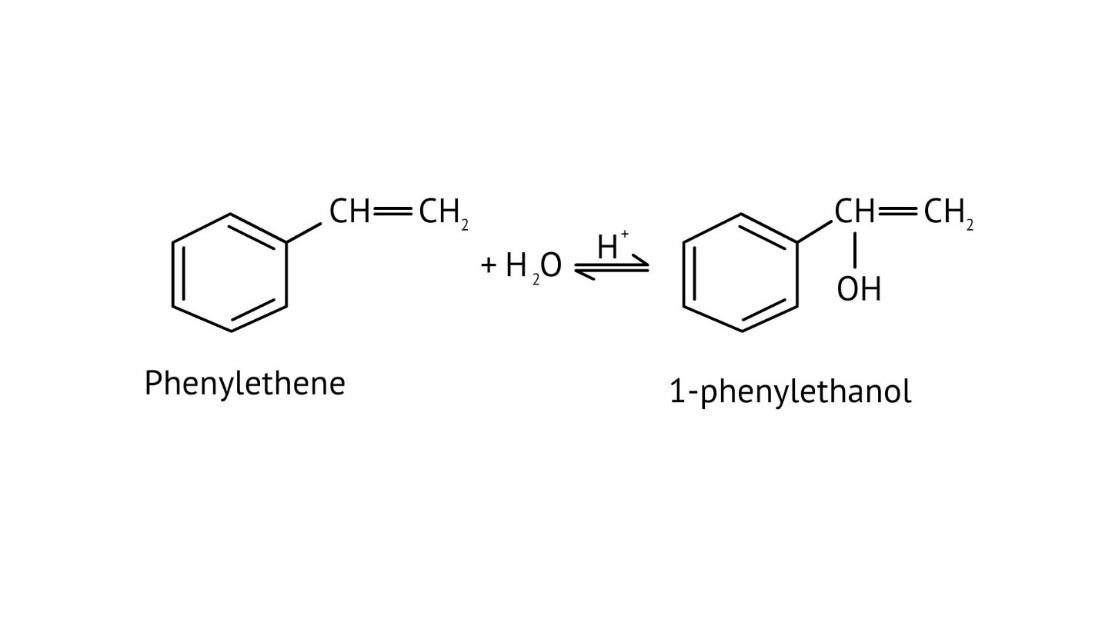
9. When solving conversion problems, how do the NCERT Solutions guide the selection of the correct reagents?
The solutions provide clear pathways for multi-step conversions. For instance, to convert propene to propan-1-ol, they guide you to use hydroboration-oxidation for anti-Markovnikov addition, rather than acid-catalysed hydration which would yield propan-2-ol. This helps in strategic reagent selection for desired products.
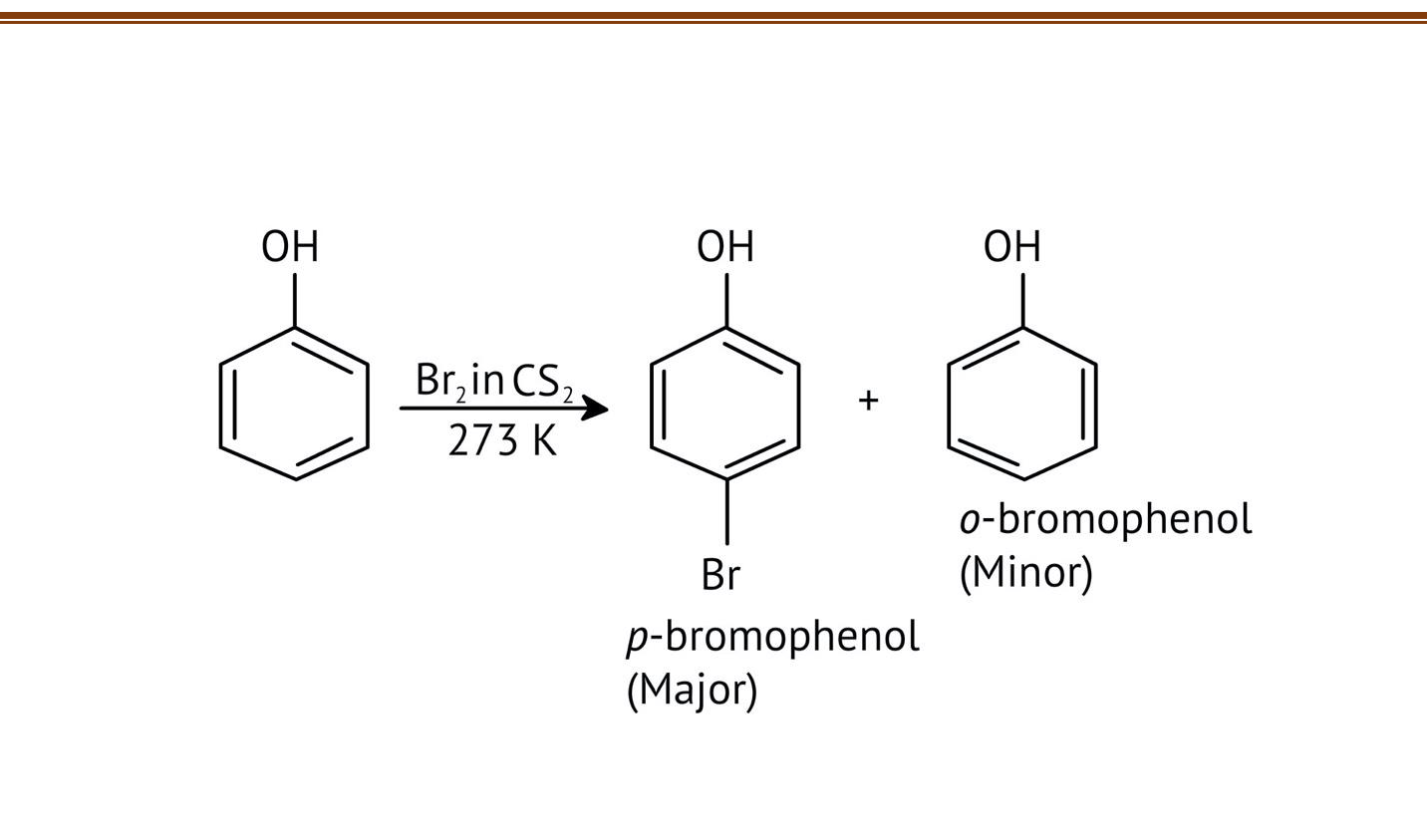
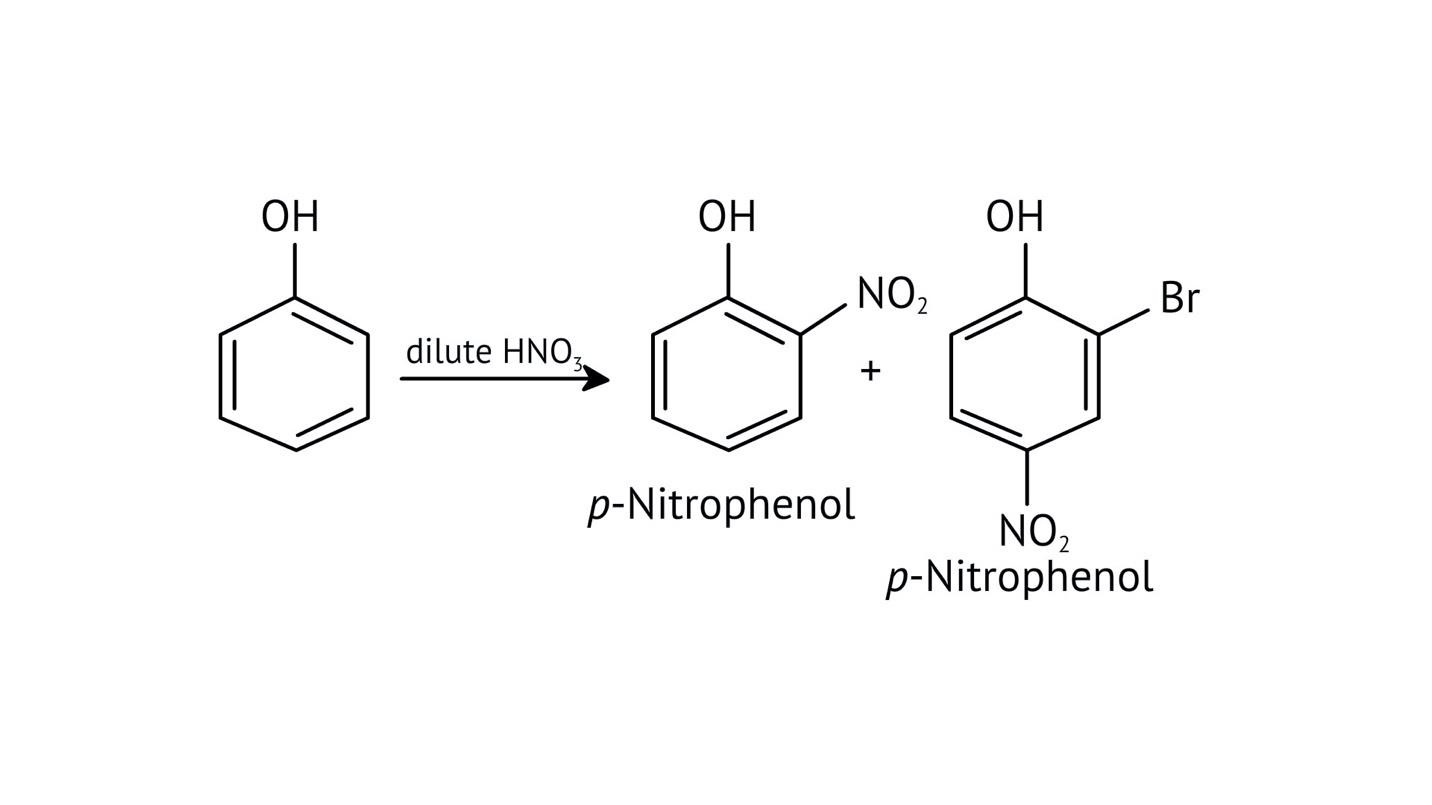
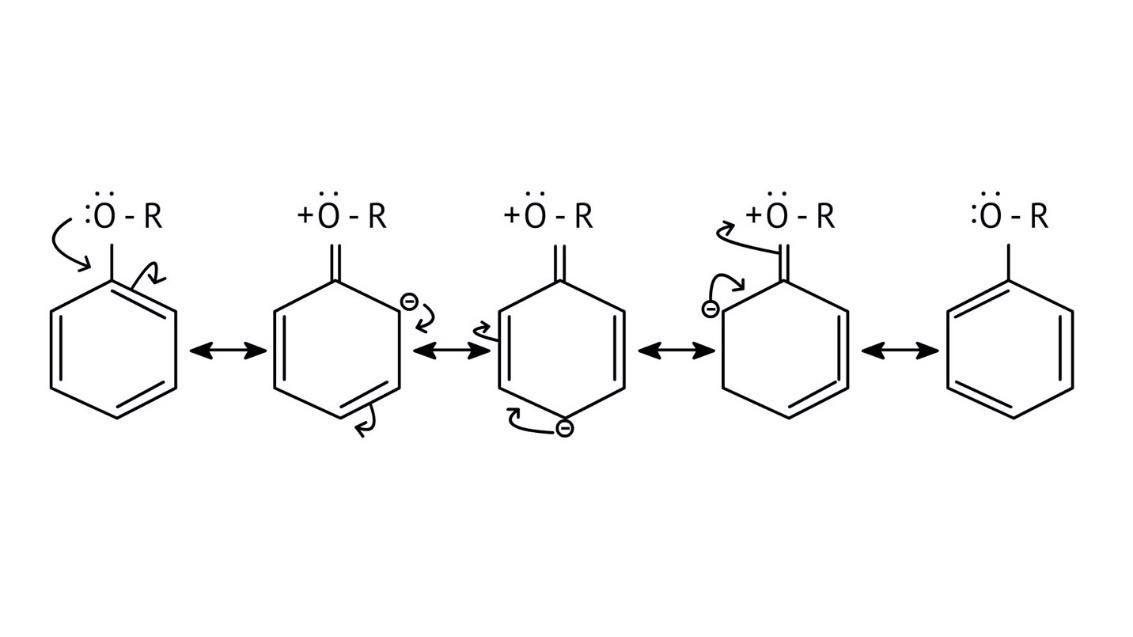
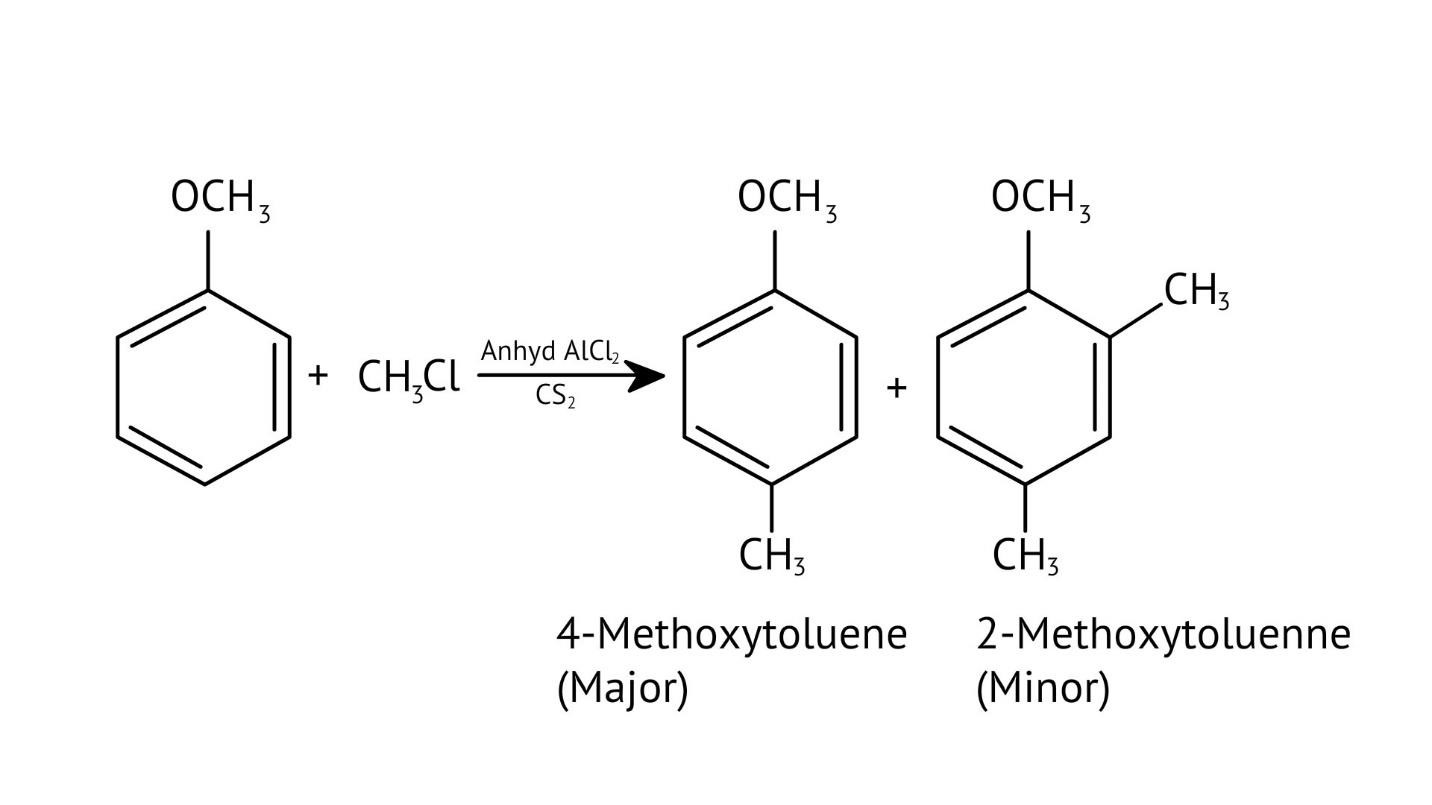
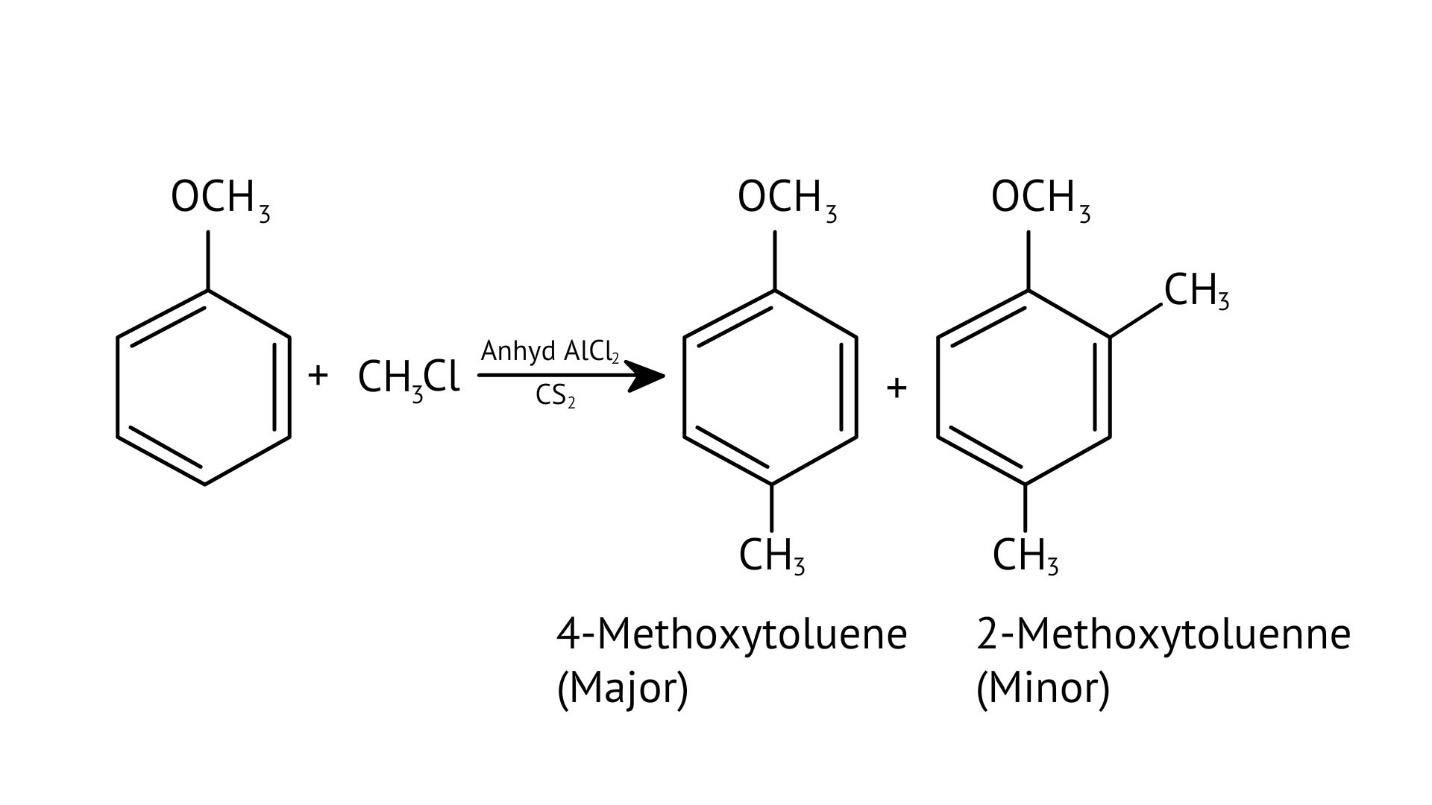
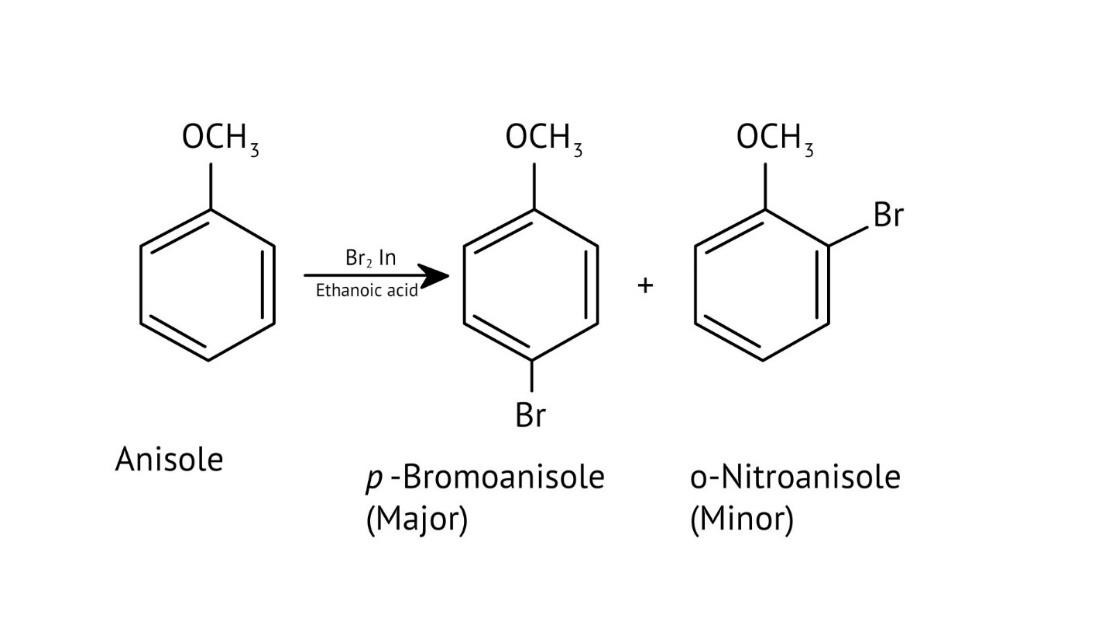
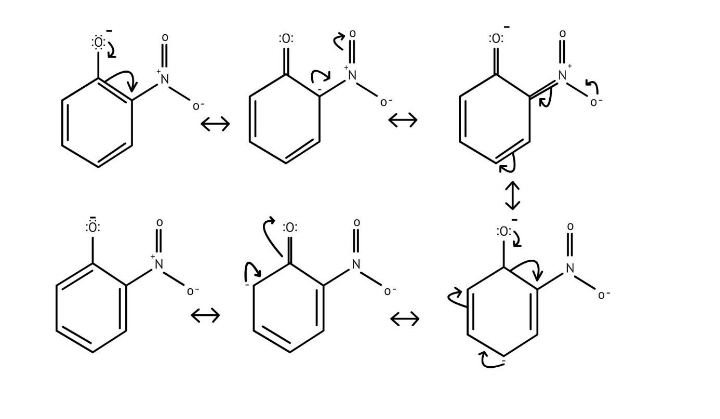
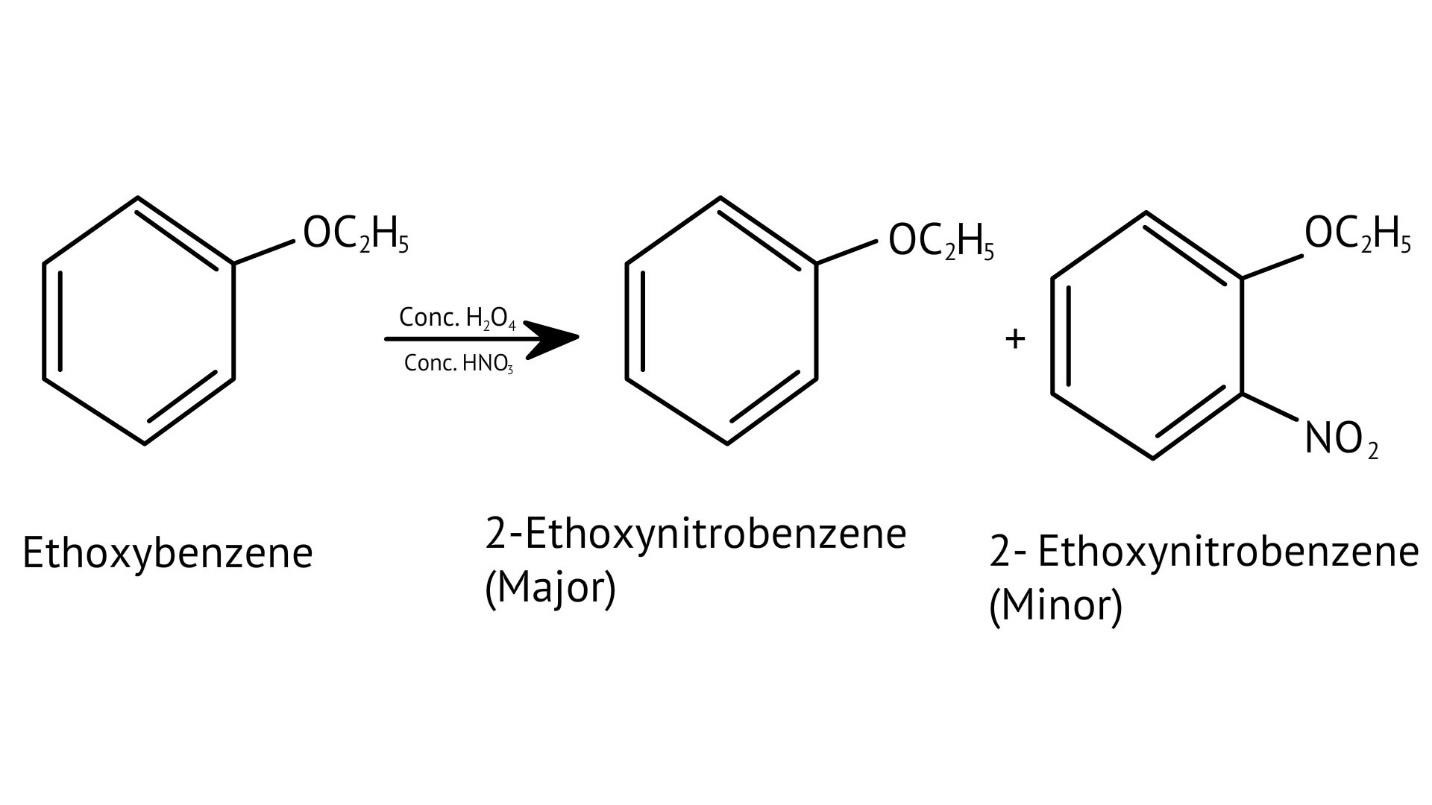
10. What is the explanation provided in the solutions for the ortho, para directing nature of the -OH group in phenol?
The solutions use resonance structures to show that the lone pairs on the oxygen of the -OH group increase electron density specifically at the ortho and para positions of the benzene ring. This activation makes these positions more susceptible to electrophilic attack, thus directing incoming electrophiles to these sites.
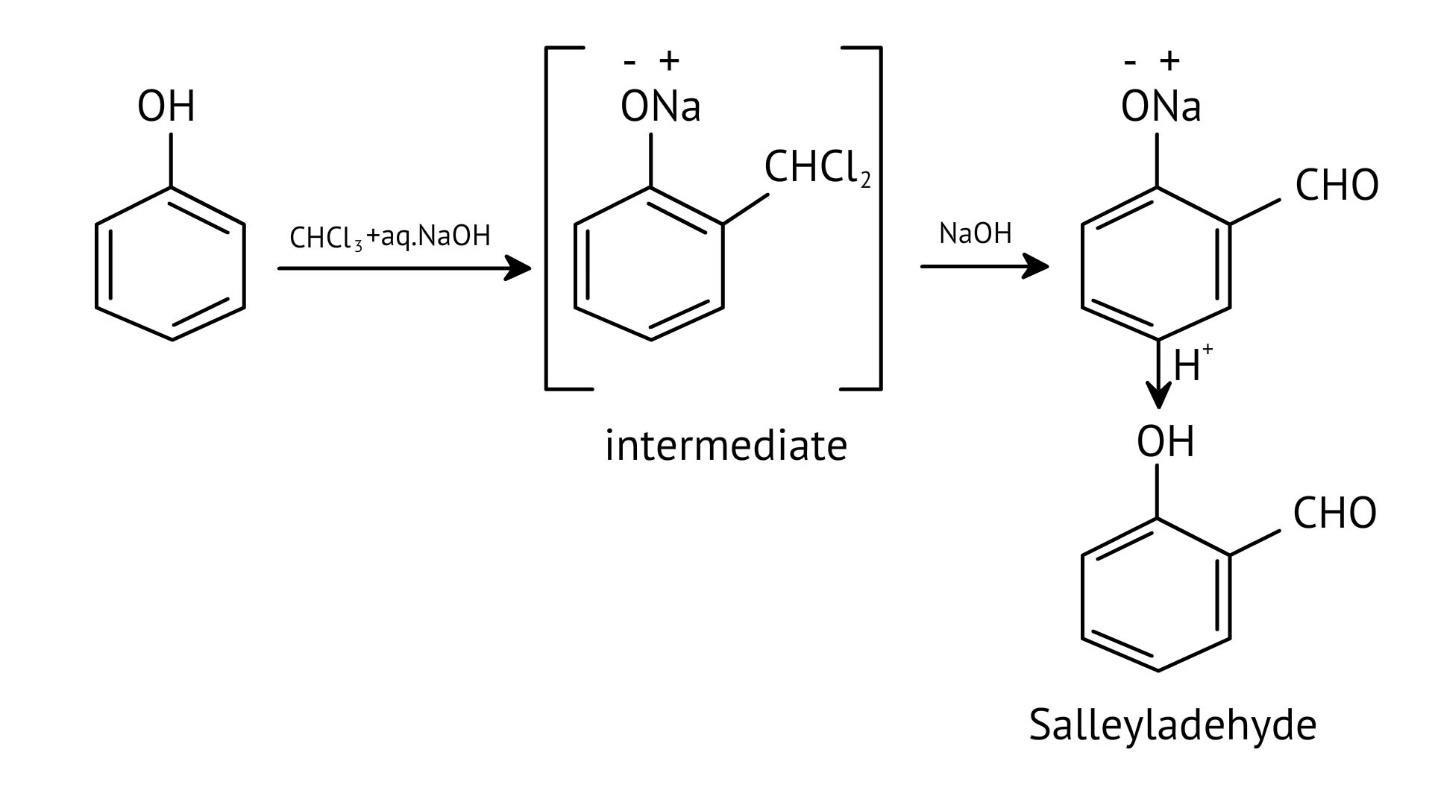
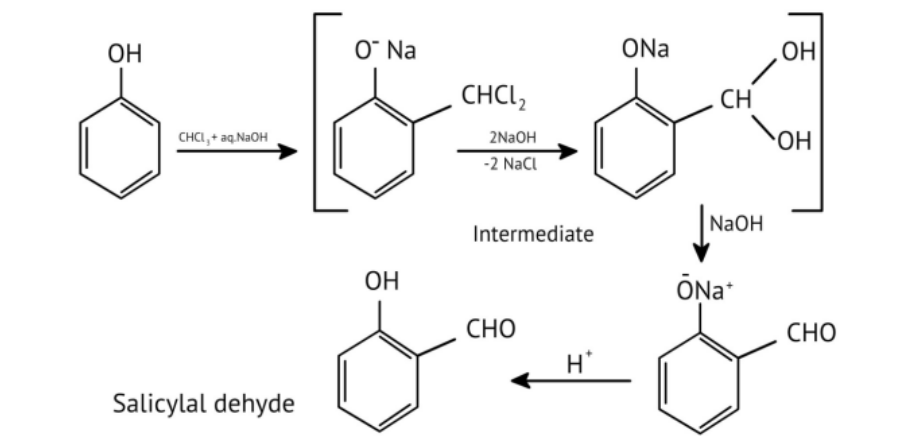
11. How do the NCERT Solutions for Chapter 7 illustrate important named reactions like the Reimer-Tiemann reaction?
The solutions illustrate the Reimer-Tiemann reaction by showing the complete chemical equation where phenol reacts with chloroform (CHCl₃) in the presence of aqueous sodium hydroxide. This process introduces a -CHO group (formyl group) at the ortho position to form salicylaldehyde, and the solutions provide the exact reagents and conditions required.